Integrated MicroRNA and mRNA Profiling in Zika Virus-Infected Neurons
- PMID: 30781519
- PMCID: PMC6410042
- DOI: 10.3390/v11020162
Integrated MicroRNA and mRNA Profiling in Zika Virus-Infected Neurons
Abstract
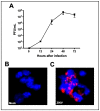
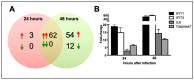
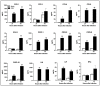
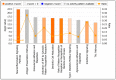
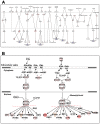
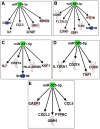
Zika virus (ZIKV) infections have caused a wide spectrum of neurological diseases, such as Guillain-Barré syndrome, myelitis, meningoencephalitis, and congenital microcephaly. No effective therapies currently exist for treating patients infected with ZIKV. MicroRNAs (miRNAs) are a group of small RNAs involved in the regulation of a wide variety of cellular and physiological processes. In this study, we analyzed digital miRNA and mRNA profiles in ZIKV-infected primary mouse neurons using the nCounter technology. A total of 599 miRNAs and 770 mRNAs were examined. We demonstrate that ZIKV infection causes global downregulation of miRNAs with only few upregulated miRNAs. ZIKV-modulated miRNAs including miR-155, miR-203, miR-29a, and miR-124-3p are known to play critical role in flavivirus infection, anti-viral immunity and brain injury. ZIKV infection also results in downregulation of miRNA processing enzymes. In contrast, ZIKV infection induces dramatic upregulation of anti-viral, inflammatory and apoptotic genes. Furthermore, our data demonstrate an inverse correlation between ZIKV-modulated miRNAs and target host mRNAs induced by ZIKV. Biofunctional analysis revealed that ZIKV-modulated miRNAs and mRNAs regulate the pathways related to neurological development and neuroinflammatory responses. Functional studies targeting specific miRNA are warranted to develop therapeutics for the management of ZIKV neurological disease.
Keywords: Zika virus; anti-viral immunity; flavivirus; microRNAs; neuroinflammation; neurons.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Cao-Lormeau V.M., Blake A., Mons S., Lastere S., Roche C., Vanhomwegen J., Dub T., Baudouin L., Teissier A., Larre P., et al. Guillain-Barré syndrome outbreak associated with zika virus infection in french polynesia: A case-control study. Lancet. 2016;387:1531–1539. doi: 10.1016/S0140-6736(16)00562-6. - DOI - PMC - PubMed
-
- Anfasa F., Siegers J.Y., van der Kroeg M., Mumtaz N., Stalin Raj V., de Vrij F.M.S., Widagdo W., Gabriel G., Salinas S., Simonin Y., et al. Phenotypic differences between asian and african lineage zika viruses in human neural progenitor cells. mSphere. 2017;2 doi: 10.1128/mSphere.00292-17. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

