Pathogenicity and Transmissibility of North American H7 Low Pathogenic Avian Influenza Viruses in Chickens and Turkeys
- PMID: 30781528
- PMCID: PMC6410290
- DOI: 10.3390/v11020163
Pathogenicity and Transmissibility of North American H7 Low Pathogenic Avian Influenza Viruses in Chickens and Turkeys
Abstract
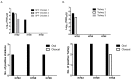
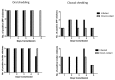
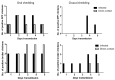
Low pathogenic avian influenza (LPAI) viruses can silently circulate in poultry and wild aquatic birds and potentially mutate into highly pathogenic avian influenza (HPAI) viruses. In the U.S., recent emergence and spread of H7N8 and H7N9 HPAI viruses not only caused devastating losses to domestic poultry but also underscored the capability of LPAI viruses to mutate into HPAI viruses. Therefore, in this study, we evaluated pathogenicity and transmissibility of H7N8 and H7N9 LPAI viruses (the progenitors of HPAI viruses) in chickens and turkeys. We also included H7N2 isolated from an outbreak of LPAI in commercial chickens. H7 viruses replicated more efficiently in the respiratory tract than in the gastrointestinal tract, suggesting that their replication is restricted to the upper respiratory tract. Specifically, H7N2 replicated most efficiently in two-week-old chickens and turkeys. In contrast, H7N8 replicated least efficiently in those birds. Further, replication of H7N2 and H7N9 was restricted in the upper respiratory tract of four-week-old specific-pathogen-free (SPF) and broiler chickens. Despite their restricted replication, the two viruses efficiently transmitted from infected to naïve birds by direct contact, leading to seroconversion of contacted chickens. Our findings suggest the importance of continuous monitoring and surveillance of LPAI viruses in the fields.
Keywords: H7; chickens; low pathogenic avian influenza virus; replication; transmissibility.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Palese P., Shaw M.L. Orthomyxoviridae: The viruses and their replication. In: Knipe D.M., Howley P.M., editors. Fields Virology. 5th ed. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2007. pp. 1647–1689.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

