Silver Nanoparticles: Synthesis and Application for Nanomedicine
- PMID: 30781560
- PMCID: PMC6412188
- DOI: 10.3390/ijms20040865
Silver Nanoparticles: Synthesis and Application for Nanomedicine
Abstract
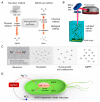
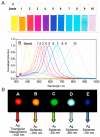
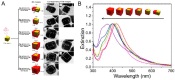
Over the past few decades, metal nanoparticles less than 100 nm in diameter have made a substantial impact across diverse biomedical applications, such as diagnostic and medical devices, for personalized healthcare practice. In particular, silver nanoparticles (AgNPs) have great potential in a broad range of applications as antimicrobial agents, biomedical device coatings, drug-delivery carriers, imaging probes, and diagnostic and optoelectronic platforms, since they have discrete physical and optical properties and biochemical functionality tailored by diverse size- and shape-controlled AgNPs. In this review, we aimed to present major routes of synthesis of AgNPs, including physical, chemical, and biological synthesis processes, along with discrete physiochemical characteristics of AgNPs. We also discuss the underlying intricate molecular mechanisms behind their plasmonic properties on mono/bimetallic structures, potential cellular/microbial cytotoxicity, and optoelectronic property. Lastly, we conclude this review with a summary of current applications of AgNPs in nanoscience and nanomedicine and discuss their future perspectives in these areas.
Keywords: characterization; cytotoxicity; diagnostics; mechanism; nanomedicine; optoelectronics; silver nanomaterial; synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Syafiuddin A., Salmiati, Salim M.R., Kueh A.B.H., Hadibarata T., Nur H. A Review of silver nanoparticles: Research trends, global consumption, synthesis, properties, and future Challenges. J. Clin. Chem. Soc. 2017;64:732–756. doi: 10.1002/jccs.201700067. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

