9-Methylfascaplysin Is a More Potent Aβ Aggregation Inhibitor than the Marine-Derived Alkaloid, Fascaplysin, and Produces Nanomolar Neuroprotective Effects in SH-SY5Y Cells
- PMID: 30781608
- PMCID: PMC6409607
- DOI: 10.3390/md17020121
9-Methylfascaplysin Is a More Potent Aβ Aggregation Inhibitor than the Marine-Derived Alkaloid, Fascaplysin, and Produces Nanomolar Neuroprotective Effects in SH-SY5Y Cells
Abstract
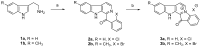
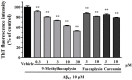
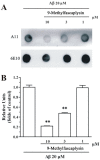
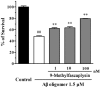
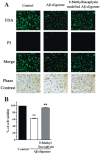
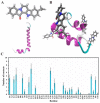
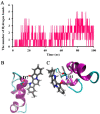
β-Amyloid (Aβ) is regarded as an important pathogenic target for Alzheimer's disease (AD), the most prevalent neurodegenerative disease. Aβ can assemble into oligomers and fibrils, and produce neurotoxicity. Therefore, Aβ aggregation inhibitors may have anti-AD therapeutic efficacies. It was found, here, that the marine-derived alkaloid, fascaplysin, inhibits Aβ fibrillization in vitro. Moreover, the new analogue, 9-methylfascaplysin, was designed and synthesized from 5-methyltryptamine. Interestingly, 9-methylfascaplysin is a more potent inhibitor of Aβ fibril formation than fascaplysin. Incubation of 9-methylfascaplysin with Aβ directly reduced Aβ oligomer formation. Molecular dynamics simulations revealed that 9-methylfascaplysin might interact with negatively charged residues of Aβ42 with polar binding energy. Hydrogen bonds and π⁻π interactions between the key amino acid residues of Aβ42 and 9-methylfascaplysin were also suggested. Most importantly, compared with the typical Aβ oligomer, Aβ modified by nanomolar 9-methylfascaplysin produced less neuronal toxicity in SH-SY5Y cells. 9-Methylfascaplysin appears to be one of the most potent marine-derived compounds that produces anti-Aβ neuroprotective effects. Given previous reports that fascaplysin inhibits acetylcholinesterase and induces P-glycoprotein, the current study results suggest that fascaplysin derivatives can be developed as novel anti-AD drugs that possibly act via inhibition of Aβ aggregation along with other target mechanisms.
Keywords: Alzheimer’s disease; Aβ; fascaplysin; oligomer; β-carboline.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
5-Hydroxycyclopenicillone Inhibits β-Amyloid Oligomerization and Produces Anti-β-Amyloid Neuroprotective Effects In Vitro.Molecules. 2017 Oct 1;22(10):1651. doi: 10.3390/molecules22101651. Molecules. 2017. PMID: 28974023 Free PMC article.
-
N-Terminus Binding Preference for Either Tanshinone or Analogue in Both Inhibition of Amyloid Aggregation and Disaggregation of Preformed Amyloid Fibrils-Toward Introducing a Kind of Novel Anti-Alzheimer Compounds.ACS Chem Neurosci. 2017 Jul 19;8(7):1577-1588. doi: 10.1021/acschemneuro.7b00080. Epub 2017 Apr 28. ACS Chem Neurosci. 2017. PMID: 28406293
-
Dimeric bis (heptyl)-Cognitin Blocks Alzheimer's β-Amyloid Neurotoxicity Via the Inhibition of Aβ Fibrils Formation and Disaggregation of Preformed Fibrils.CNS Neurosci Ther. 2015 Dec;21(12):953-61. doi: 10.1111/cns.12472. Epub 2015 Oct 28. CNS Neurosci Ther. 2015. PMID: 26507365 Free PMC article.
-
Elucidating the Structures of Amyloid Oligomers with Macrocyclic β-Hairpin Peptides: Insights into Alzheimer's Disease and Other Amyloid Diseases.Acc Chem Res. 2018 Mar 20;51(3):706-718. doi: 10.1021/acs.accounts.7b00554. Epub 2018 Mar 6. Acc Chem Res. 2018. PMID: 29508987 Free PMC article. Review.
-
Understanding amyloid fibril nucleation and aβ oligomer/drug interactions from computer simulations.Acc Chem Res. 2014 Feb 18;47(2):603-11. doi: 10.1021/ar4002075. Epub 2013 Dec 24. Acc Chem Res. 2014. PMID: 24368046 Review.
Cited by
-
A New Mild Method for Synthesis of Marine Alkaloid Fascaplysin and Its Therapeutically Promising Derivatives.Mar Drugs. 2023 Jul 25;21(8):424. doi: 10.3390/md21080424. Mar Drugs. 2023. PMID: 37623705 Free PMC article.
-
Bisindole Compounds-Synthesis and Medicinal Properties.Antibiotics (Basel). 2024 Dec 13;13(12):1212. doi: 10.3390/antibiotics13121212. Antibiotics (Basel). 2024. PMID: 39766602 Free PMC article. Review.
-
Marine-Derived Lead Fascaplysin: Pharmacological Activity, Total Synthesis, and Structural Modification.Mar Drugs. 2023 Mar 31;21(4):226. doi: 10.3390/md21040226. Mar Drugs. 2023. PMID: 37103365 Free PMC article. Review.
-
Marine Natural Products from the Russian Pacific as Sources of Drugs for Neurodegenerative Diseases.Mar Drugs. 2022 Nov 11;20(11):708. doi: 10.3390/md20110708. Mar Drugs. 2022. PMID: 36421986 Free PMC article. Review.
-
Total Syntheses and Preliminary Biological Evaluation of Brominated Fascaplysin and Reticulatine Alkaloids and Their Analogues.Mar Drugs. 2019 Aug 25;17(9):496. doi: 10.3390/md17090496. Mar Drugs. 2019. PMID: 31450717 Free PMC article.
References
-
- Ulep M.G., Saraon S.K., McLea S. Alzheimer Disease. J. Nurse Pract. 2018;14:129–135. doi: 10.1016/j.nurpra.2017.10.014. - DOI
MeSH terms
Substances
Grants and funding
- 81870853, 21576199, 21878234, 20903058, 81850410553/National Natural Science Foundation of China
- 2017C50042/Ningbo Sci & Tech Project for Common Wealth
- 201804/Zhejiang Key Laboratory of Pathophysiology
- SKLFNS-KF-201806/Open Project Program of State Key Laboratory of Food Nutrition and Safety, Tianjin University of Science & Technology
- 2015C110026/Ningbo municipal innovation team of life science and health
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

