A Smart Nanovector for Cancer Targeted Drug Delivery Based on Graphene Quantum Dots
- PMID: 30781623
- PMCID: PMC6409783
- DOI: 10.3390/nano9020282
A Smart Nanovector for Cancer Targeted Drug Delivery Based on Graphene Quantum Dots
Abstract
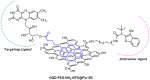
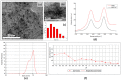
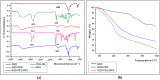
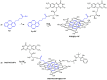
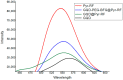
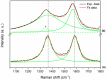
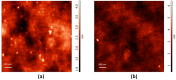
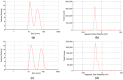
Graphene quantum dots (GQD), the new generation members of graphene-family, have shown promising applications in anticancer therapy. In this study, we report the synthesis of a fluorescent and biocompatible nanovector, based on GQD, for the targeted delivery of an anticancer drug with benzofuran structure (BFG) and bearing the targeting ligand riboflavin (RF, vitamin B2). The highly water-dispersible nanoparticles, synthesized from multi-walled carbon nanotubes (MWCNT) by prolonged acidic treatment, were linked covalently to the drug by means of a cleavable PEG linker while the targeting ligand RF was conjugated to the GQD by π⁻π interaction using a pyrene linker. The cytotoxic effect of the synthesized drug delivery system (DDS) GQD-PEG-BFG@Pyr-RF was tested on three cancer cell lines and this effect was compared with that exerted by the same nanovector lacking the RF ligand (GQD-PEG-BFG) or the anticancer drug (GQD@Pyr-RF). The results of biological tests underlined the low cytotoxicity of the GQD sample and the cytotoxic activity of the DDS against the investigated cancer cell lines with a higher or similar potency to that exerted by the BFG alone, thus opening new possibilities for the use of this drug or other anticancer agents endowed of cytotoxicity and serious side effects.
Keywords: anticancer therapy; drug delivery systems; graphene quantum dots.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Graphene quantum dots for cancer targeted drug delivery.Int J Pharm. 2017 Feb 25;518(1-2):185-192. doi: 10.1016/j.ijpharm.2016.12.060. Epub 2017 Jan 2. Int J Pharm. 2017. PMID: 28057464
-
Graphene Quantum Dots Based Systems As HIV Inhibitors.Bioconjug Chem. 2018 Sep 19;29(9):3084-3093. doi: 10.1021/acs.bioconjchem.8b00448. Epub 2018 Aug 22. Bioconjug Chem. 2018. PMID: 30106563
-
Two-photon photodynamic therapy based on FRET using tumor-cell targeted riboflavin conjugated graphene quantum dot.J Photochem Photobiol B. 2023 Jan;238:112602. doi: 10.1016/j.jphotobiol.2022.112602. Epub 2022 Nov 23. J Photochem Photobiol B. 2023. PMID: 36442423
-
Bioactive Graphene Quantum Dots Based Polymer Composite for Biomedical Applications.Polymers (Basel). 2022 Feb 5;14(3):617. doi: 10.3390/polym14030617. Polymers (Basel). 2022. PMID: 35160606 Free PMC article. Review.
-
A review of graphene quantum dots and their potential biomedical applications.J Biomater Appl. 2023 Feb;37(7):1137-1158. doi: 10.1177/08853282221125311. Epub 2022 Sep 6. J Biomater Appl. 2023. PMID: 36066191 Review.
Cited by
-
Graphene Quantum Dots as Flourishing Nanomaterials for Bio-Imaging, Therapy Development, and Micro-Supercapacitors.Micromachines (Basel). 2020 Sep 18;11(9):866. doi: 10.3390/mi11090866. Micromachines (Basel). 2020. PMID: 32962061 Free PMC article. Review.
-
Biological Potential of Polyethylene Glycol (PEG)-Functionalized Graphene Quantum Dots in In Vitro Neural Stem/Progenitor Cells.Nanomaterials (Basel). 2021 May 29;11(6):1446. doi: 10.3390/nano11061446. Nanomaterials (Basel). 2021. PMID: 34072613 Free PMC article.
-
Biomedical Applications of Quantum Dots: Overview, Challenges, and Clinical Potential.Int J Nanomedicine. 2022 May 2;17:1951-1970. doi: 10.2147/IJN.S357980. eCollection 2022. Int J Nanomedicine. 2022. PMID: 35530976 Free PMC article. Review.
-
Use of graphene-based materials as carriers of bioactive agents.Asian J Pharm Sci. 2021 Sep;16(5):577-588. doi: 10.1016/j.ajps.2020.11.004. Epub 2020 Dec 7. Asian J Pharm Sci. 2021. PMID: 34849163 Free PMC article. Review.
-
PrPC Aptamer Conjugated-Gold Nanoparticles for Targeted Delivery of Doxorubicin to Colorectal Cancer Cells.Int J Mol Sci. 2021 Feb 17;22(4):1976. doi: 10.3390/ijms22041976. Int J Mol Sci. 2021. PMID: 33671292 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

