Loss of C/EBPδ Exacerbates Radiation-Induced Cognitive Decline in Aged Mice due to Impaired Oxidative Stress Response
- PMID: 30781689
- PMCID: PMC6412914
- DOI: 10.3390/ijms20040885
Loss of C/EBPδ Exacerbates Radiation-Induced Cognitive Decline in Aged Mice due to Impaired Oxidative Stress Response
Abstract
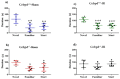
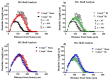
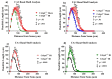
Aging is characterized by increased inflammation and deterioration of the cellular stress responses such as the oxidant/antioxidant equilibrium, DNA damage repair fidelity, and telomeric attrition. All these factors contribute to the increased radiation sensitivity in the elderly as shown by epidemiological studies of the Japanese atomic bomb survivors. There is a global increase in the aging population, who may be at increased risk of exposure to ionizing radiation (IR) as part of cancer therapy or accidental exposure. Therefore, it is critical to delineate the factors that exacerbate age-related radiation sensitivity and neurocognitive decline. The transcription factor CCAAT enhancer binding protein delta (C/EBPδ) is implicated with regulatory roles in neuroinflammation, learning, and memory, however its role in IR-induced neurocognitive decline and aging is not known. The purpose of this study was to delineate the role of C/EBPδ in IR-induced neurocognitive decline in aged mice. We report that aged Cebpd-/- mice exposed to acute IR exposure display impairment in short-term memory and spatial memory that correlated with significant alterations in the morphology of neurons in the dentate gyrus (DG) and CA1 apical and basal regions. There were no significant changes in the expression of inflammatory markers. However, the expression of superoxide dismutase 2 (SOD2) and catalase (CAT) were altered post-IR in the hippocampus of aged Cebpd-/- mice. These results suggest that Cebpd may protect from IR-induced neurocognitive dysfunction by suppressing oxidative stress in aged mice.
Keywords: C/EBPδ; Cebpd; behavior; hippocampus; ionizing radiation; novel object recognition; oxidative stress; reactive oxygen species; short-term memory; spatial learning.
Conflict of interest statement
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; and in the decision to publish the results.
Figures




Similar articles
-
Loss of C/EBPδ enhances IR-induced cell death by promoting oxidative stress and mitochondrial dysfunction.Free Radic Biol Med. 2016 Oct;99:296-307. doi: 10.1016/j.freeradbiomed.2016.08.022. Epub 2016 Aug 20. Free Radic Biol Med. 2016. PMID: 27554969 Free PMC article.
-
C/EBPδ deficiency sensitizes mice to ionizing radiation-induced hematopoietic and intestinal injury.PLoS One. 2014 Apr 18;9(4):e94967. doi: 10.1371/journal.pone.0094967. eCollection 2014. PLoS One. 2014. PMID: 24747529 Free PMC article.
-
C/EBPδ protects from radiation-induced intestinal injury and sepsis by suppression of inflammatory and nitrosative stress.Sci Rep. 2019 Sep 27;9(1):13953. doi: 10.1038/s41598-019-49437-x. Sci Rep. 2019. PMID: 31562350 Free PMC article.
-
C/EBPβ and C/EBPδ transcription factors: Basic biology and roles in the CNS.Prog Neurobiol. 2015 Sep;132:1-33. doi: 10.1016/j.pneurobio.2015.06.003. Epub 2015 Jul 2. Prog Neurobiol. 2015. PMID: 26143335 Review.
-
Central Nervous System Response Against Ionizing Radiation Exposure: Cellular, Biochemical, and Molecular Perspectives.Mol Neurobiol. 2025 Jun;62(6):7268-7295. doi: 10.1007/s12035-025-04712-z. Epub 2025 Jan 28. Mol Neurobiol. 2025. PMID: 39875779 Review.
Cited by
-
A Single-Cell Transcriptomic Analysis of the Mouse Hippocampus After Voluntary Exercise.Mol Neurobiol. 2024 Aug;61(8):5628-5645. doi: 10.1007/s12035-023-03869-9. Epub 2024 Jan 13. Mol Neurobiol. 2024. PMID: 38217668 Free PMC article.
-
CCAAT/Enhancer-Binding Protein Delta Regulates Glioblastoma Survival through Catalase-Mediated Hydrogen Peroxide Clearance.Oxid Med Cell Longev. 2022 Aug 18;2022:4081380. doi: 10.1155/2022/4081380. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36035213 Free PMC article.
-
Gpx3 and Egr1 Are Involved in Regulating the Differentiation Fate of Cardiac Fibroblasts under Pressure Overload.Oxid Med Cell Longev. 2022 Jun 28;2022:3235250. doi: 10.1155/2022/3235250. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35799890 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

