RHOG Activates RAC1 through CDC42 Leading to Tube Formation in Vascular Endothelial Cells
- PMID: 30781697
- PMCID: PMC6406863
- DOI: 10.3390/cells8020171
RHOG Activates RAC1 through CDC42 Leading to Tube Formation in Vascular Endothelial Cells
Abstract
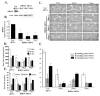
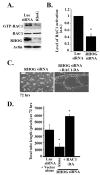
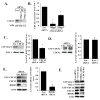
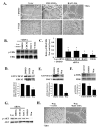
Angiogenesis is a hallmark of cancer cell malignancy. The role of the RHO family GTPase RHOG in angiogenesis in vascular endothelial cells has recently been elucidated. However, the regulation of RHOG during this process, as well as its cross-talk with other RHO GTPases, have yet to be fully examined. In this study, we found that siRNA-mediated depletion of RHOG strongly inhibits tube formation in vascular endothelial cells (ECV cells), an effect reversed by transfecting dominant active constructs of CDC42 or RAC1 in the RHOG-depleted cells. We also found CDC42 to be upstream from RAC1 in these cells. Inhibiting either Phosphatidyl inositol (3) kinase (PI3K) with Wortmannin or the mitogen-activated protein kinase extracellular-regulated kinase (MAPK ERK) with U0126 leads to the inhibition of tube formation. While knocking down either RHO, GTPase did not affect p-AKT levels, and p-ERK decreased in response to the knocking down of RHOG, CDC42 or RAC1. Recovering active RHO GTPases in U0126-treated cells also did not reverse the inhibition of tube formation, placing ERK downstream from PI3K-RHOG-CDC42-RAC1 in vascular endothelial cells. Finally, RHOA and the Rho activated protein kinases ROCK1 and ROCK2 positively regulated tube formation independently of ERK, while RHOC seemed to inhibit the process. Collectively, our data confirmed the essential role of RHOG in angiogenesis, shedding light on a potential new therapeutic target for cancer malignancy and metastasis.
Keywords: CDC42; RAC1; RHO GTPases; RHOG; angiogenesis; vascular endothelial cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
RhoG signals in parallel with Rac1 and Cdc42.J Biol Chem. 2002 Dec 6;277(49):47810-7. doi: 10.1074/jbc.M203816200. Epub 2002 Oct 9. J Biol Chem. 2002. PMID: 12376551
-
Different roles of the small GTPases Rac1, Cdc42, and RhoG in CALEB/NGC-induced dendritic tree complexity.J Neurochem. 2016 Oct;139(1):26-39. doi: 10.1111/jnc.13735. Epub 2016 Aug 4. J Neurochem. 2016. PMID: 27412363
-
Role of Rac1 and Cdc42 in hypoxia induced p53 and von Hippel-Lindau suppression and HIF1alpha activation.Int J Cancer. 2006 Jun 15;118(12):2965-72. doi: 10.1002/ijc.21763. Int J Cancer. 2006. PMID: 16395716
-
Driving Rho GTPase activity in endothelial cells regulates barrier integrity.Thromb Haemost. 2010 Jan;103(1):40-55. doi: 10.1160/TH09-06-0403. Epub 2009 Sep 30. Thromb Haemost. 2010. PMID: 20062930 Review.
-
The role of Rho GTPases' substrates Rac and Cdc42 in osteoclastogenesis and relevant natural medicinal products study.Biosci Rep. 2020 Jul 31;40(7):BSR20200407. doi: 10.1042/BSR20200407. Biosci Rep. 2020. PMID: 32578854 Free PMC article. Review.
Cited by
-
URB597 and Andrographolide Improve Brain Microvascular Endothelial Cell Permeability and Apoptosis by Reducing Oxidative Stress and Inflammation Associated with Activation of Nrf2 Signaling in Oxygen-Glucose Deprivation.Oxid Med Cell Longev. 2022 May 12;2022:4139330. doi: 10.1155/2022/4139330. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35602108 Free PMC article.
-
Differential regulation of rho GTPases during lung adenocarcinoma migration and invasion reveals a novel role of the tumor suppressor StarD13 in invadopodia regulation.Cell Commun Signal. 2020 Sep 8;18(1):144. doi: 10.1186/s12964-020-00635-5. Cell Commun Signal. 2020. PMID: 32900380 Free PMC article.
-
A multiscale whole-cell theory for mechanosensitive migration on viscoelastic substrates.Biophys J. 2023 Jan 3;122(1):114-129. doi: 10.1016/j.bpj.2022.11.022. Epub 2022 Dec 8. Biophys J. 2023. PMID: 36493781 Free PMC article.
-
Human Recombinant Arginase I [HuArgI (Co)-PEG5000]-Induced Arginine Depletion Inhibits Colorectal Cancer Cell Migration and Invasion.Int J Mol Sci. 2019 Nov 29;20(23):6018. doi: 10.3390/ijms20236018. Int J Mol Sci. 2019. PMID: 31795337 Free PMC article.
-
FOXK2 transcription factor and its roles in tumorigenesis (Review).Oncol Lett. 2022 Nov 3;24(6):461. doi: 10.3892/ol.2022.13581. eCollection 2022 Dec. Oncol Lett. 2022. PMID: 36380871 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

