Chronic Hypoxia Enhances β-Oxidation-Dependent Electron Transport via Electron Transferring Flavoproteins
- PMID: 30781698
- PMCID: PMC6406996
- DOI: 10.3390/cells8020172
Chronic Hypoxia Enhances β-Oxidation-Dependent Electron Transport via Electron Transferring Flavoproteins
Abstract
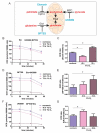
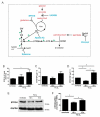
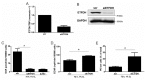
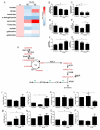
Hypoxia poses a stress to cells and decreases mitochondrial respiration, in part by electron transport chain (ETC) complex reorganization. While metabolism under acute hypoxia is well characterized, alterations under chronic hypoxia largely remain unexplored. We followed oxygen consumption rates in THP-1 monocytes during acute (16 h) and chronic (72 h) hypoxia, compared to normoxia, to analyze the electron flows associated with glycolysis, glutamine, and fatty acid oxidation. Oxygen consumption under acute hypoxia predominantly demanded pyruvate, while under chronic hypoxia, fatty acid- and glutamine-oxidation dominated. Chronic hypoxia also elevated electron-transferring flavoproteins (ETF), and the knockdown of ETF⁻ubiquinone oxidoreductase lowered mitochondrial respiration under chronic hypoxia. Metabolomics revealed an increase in citrate under chronic hypoxia, which implied glutamine processing to α-ketoglutarate and citrate. Expression regulation of enzymes involved in this metabolic shunting corroborated this assumption. Moreover, the expression of acetyl-CoA carboxylase 1 increased, thus pointing to fatty acid synthesis under chronic hypoxia. Cells lacking complex I, which experienced a markedly impaired respiration under normoxia, also shifted their metabolism to fatty acid-dependent synthesis and usage. Taken together, we provide evidence that chronic hypoxia fuels the ETC via ETFs, increasing fatty acid production and consumption via the glutamine-citrate-fatty acid axis.
Keywords: TMEM126B; complex I; electron transport chain; fatty acids; glutamine; mitochondria; monocytes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Electron transport chain-dependent and -independent mechanisms of mitochondrial H2O2 emission during long-chain fatty acid oxidation.J Biol Chem. 2010 Feb 19;285(8):5748-58. doi: 10.1074/jbc.M109.026203. Epub 2009 Dec 23. J Biol Chem. 2010. PMID: 20032466 Free PMC article.
-
Coenzyme Q10 serves to couple mitochondrial oxidative phosphorylation and fatty acid β-oxidation, and attenuates NLRP3 inflammasome activation.Free Radic Res. 2018 Dec;52(11-12):1445-1455. doi: 10.1080/10715762.2018.1500695. Epub 2018 Sep 11. Free Radic Res. 2018. PMID: 30003820
-
Structure of electron transfer flavoprotein-ubiquinone oxidoreductase and electron transfer to the mitochondrial ubiquinone pool.Proc Natl Acad Sci U S A. 2006 Oct 31;103(44):16212-7. doi: 10.1073/pnas.0604567103. Epub 2006 Oct 18. Proc Natl Acad Sci U S A. 2006. PMID: 17050691 Free PMC article.
-
The inborn errors of mitochondrial fatty acid oxidation.J Inherit Metab Dis. 1987;10 Suppl 1:159-200. doi: 10.1007/BF01812855. J Inherit Metab Dis. 1987. PMID: 3119938 Review.
-
The electron transfer flavoprotein: ubiquinone oxidoreductases.Biochim Biophys Acta. 2010 Dec;1797(12):1910-6. doi: 10.1016/j.bbabio.2010.10.007. Epub 2010 Oct 16. Biochim Biophys Acta. 2010. PMID: 20937244 Review.
Cited by
-
Considerations about Hypoxic Changes in Neuraxis Tissue Injuries and Recovery.Biomedicines. 2022 Feb 18;10(2):481. doi: 10.3390/biomedicines10020481. Biomedicines. 2022. PMID: 35203690 Free PMC article. Review.
-
Distinct metabolic hallmarks of WHO classified adult glioma subtypes.Neuro Oncol. 2022 Sep 1;24(9):1454-1468. doi: 10.1093/neuonc/noac042. Neuro Oncol. 2022. PMID: 35157758 Free PMC article.
-
Immune escape and metastasis mechanisms in melanoma: breaking down the dichotomy.Front Immunol. 2024 Feb 14;15:1336023. doi: 10.3389/fimmu.2024.1336023. eCollection 2024. Front Immunol. 2024. PMID: 38426087 Free PMC article. Review.
-
Functional states of myeloid cells in cancer.Cancer Cell. 2023 Mar 13;41(3):490-504. doi: 10.1016/j.ccell.2023.02.009. Epub 2023 Mar 2. Cancer Cell. 2023. PMID: 36868224 Free PMC article. Review.
-
BCR::ABL1 expression in chronic myeloid leukemia cells in low oxygen is regulated by glutamine via CD36-mediated fatty acid uptake.Cancer Cell Int. 2025 May 14;25(1):176. doi: 10.1186/s12935-025-03805-y. Cancer Cell Int. 2025. PMID: 40369538 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

