The Functional Implications of Endothelial Gap Junctions and Cellular Mechanics in Vascular Angiogenesis
- PMID: 30781714
- PMCID: PMC6406946
- DOI: 10.3390/cancers11020237
The Functional Implications of Endothelial Gap Junctions and Cellular Mechanics in Vascular Angiogenesis
Abstract
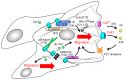
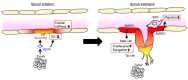
Angiogenesis-the sprouting and growth of new blood vessels from the existing vasculature-is an important contributor to tumor development, since it facilitates the supply of oxygen and nutrients to cancer cells. Endothelial cells are critically affected during the angiogenic process as their proliferation, motility, and morphology are modulated by pro-angiogenic and environmental factors associated with tumor tissues and cancer cells. Recent in vivo and in vitro studies have revealed that the gap junctions of endothelial cells also participate in the promotion of angiogenesis. Pro-angiogenic factors modulate gap junction function and connexin expression in endothelial cells, whereas endothelial connexins are involved in angiogenic tube formation and in the cell migration of endothelial cells. Several mechanisms, including gap junction function-dependent or -independent pathways, have been proposed. In particular, connexins might have the potential to regulate cell mechanics such as cell morphology, cell migration, and cellular stiffness that are dynamically changed during the angiogenic processes. Here, we review the implication for endothelial gap junctions and cellular mechanics in vascular angiogenesis.
Keywords: angiogenesis; cell mechanics; cell migration; cellular stiffness; connexin; gap junction.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Endothelial connexin32 enhances angiogenesis by positively regulating tube formation and cell migration.Exp Cell Res. 2014 Feb 15;321(2):133-41. doi: 10.1016/j.yexcr.2013.12.002. Epub 2013 Dec 11. Exp Cell Res. 2014. PMID: 24333598
-
Connexins: Key Players in the Control of Vascular Plasticity and Function.Physiol Rev. 2020 Apr 1;100(2):525-572. doi: 10.1152/physrev.00010.2019. Physiol Rev. 2020. PMID: 31939708 Review.
-
Communication between malignant glioma cells and vascular endothelial cells through gap junctions.J Neurosurg. 2003 Apr;98(4):846-53. doi: 10.3171/jns.2003.98.4.0846. J Neurosurg. 2003. PMID: 12691411
-
Bioglass promotes wound healing by affecting gap junction connexin 43 mediated endothelial cell behavior.Biomaterials. 2016 Apr;84:64-75. doi: 10.1016/j.biomaterials.2016.01.033. Epub 2016 Jan 19. Biomaterials. 2016. PMID: 26821121
-
Endothelial connexins in vascular function.Vasc Biol. 2019 Nov 7;1(1):H117-H124. doi: 10.1530/VB-19-0015. eCollection 2019. Vasc Biol. 2019. PMID: 32923963 Free PMC article. Review.
Cited by
-
Antagonistic Functions of Connexin 43 during the Development of Primary or Secondary Bone Tumors.Biomolecules. 2020 Aug 26;10(9):1240. doi: 10.3390/biom10091240. Biomolecules. 2020. PMID: 32859065 Free PMC article. Review.
-
The Role of Connexin in Ophthalmic Neovascularization and the Interaction between Connexin and Proangiogenic Factors.J Ophthalmol. 2022 Jun 22;2022:8105229. doi: 10.1155/2022/8105229. eCollection 2022. J Ophthalmol. 2022. PMID: 35783340 Free PMC article. Review.
-
Gap Junctions and Connexins in Cancer Formation, Progression, and Therapy.Cancers (Basel). 2020 Nov 9;12(11):3307. doi: 10.3390/cancers12113307. Cancers (Basel). 2020. PMID: 33182480 Free PMC article. No abstract available.
-
A DNA Vaccine Against Proadrenomedullin N-Terminal 20 Peptide (PAMP) Reduces Angiogenesis and Increases Lymphocyte and Macrophage Infiltration but Has No Effect on Tumor Burden in a Mouse Model of Lung Metastasis.Vaccines (Basel). 2025 May 30;13(6):586. doi: 10.3390/vaccines13060586. Vaccines (Basel). 2025. PMID: 40573917 Free PMC article.
-
Regulation of angiogenesis by microRNAs in cancer.Mol Med Rep. 2021 Aug;24(2):583. doi: 10.3892/mmr.2021.12222. Epub 2021 Jun 16. Mol Med Rep. 2021. PMID: 34132365 Free PMC article. Review.
References
-
- Jakobsson L., Franco C.A., Bentley K., Collins R.T., Ponsioen B., Aspalter I.M., Rosewell I., Busse M., Thurston G., Medvinsky A., et al. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat. Cell Biol. 2010;12:943–953. doi: 10.1038/ncb2103. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

