Preventive Effect of YGDEY from Tilapia Fish Skin Gelatin Hydrolysates against Alcohol-Induced Damage in HepG2 Cells through ROS-Mediated Signaling Pathways
- PMID: 30781878
- PMCID: PMC6412572
- DOI: 10.3390/nu11020392
Preventive Effect of YGDEY from Tilapia Fish Skin Gelatin Hydrolysates against Alcohol-Induced Damage in HepG2 Cells through ROS-Mediated Signaling Pathways
Abstract
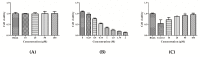
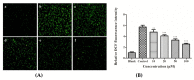
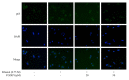
According to a previous study, YGDEY from tilapia fish skin gelatin hydrolysates has strong free radical scavenging activity. In the present study, the protective effect of YGDEY against oxidative stress induced by ethanol in HepG2 cells was investigated. First, cells were incubated with YGDEY (10, 20, 50, and 100 μM) to assess cytotoxicity, and there was no significant change in cell viability. Next, it was established that YGDEY decreased the production of reactive oxygen species (ROS). Western blot results indicated that YGDEY increased the levels of superoxide dismutase (SOD) and glutathione (GSH) and decreased the expression of gamma-glutamyltransferase (GGT) in HepG2 cells. It was then revealed that YGDEY markedly reduced the expressions of bax and cleaved-caspase-3 (c-caspase-3); inhibited phosphorylation of Akt, IκB-α, p65, and p38; and increased the level of bcl-2. Moreover, the comet assay showed that YGDEY effectively decreased the amount of ethanol-induced DNA damage. Thus, YGDEY protected HepG2 cells from alcohol-induced injury by inhibiting oxidative stress, and this may be associated with the Akt/nuclear factor-κB (NF-κB)/mitogen-activated protein kinase (MAPK) signal transduction pathways. These results demonstrate that YGDEY from tilapia fish skin gelatin hydrolysates protects HepG2 cells from oxidative stress, making it a potential functional food ingredient.
Keywords: Alcohol metabolism; DNA damage; HepG2 cells; Oxidative stress; YGDEY.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Liu Y.G., Wang J., Li L.Z., Hu W.J., Qu Y.D., Ding Y.P., Meng L.N., Teng L.R., Wang D. Hepatoprotective effects of Antrodia cinnamomea: The modulation of oxidative stress signaling in a mouse model of alcohol-induced acute liver injury. Oxid. Med. Cell. Longev. 2017:7841823. doi: 10.1155/2017/7841823. - DOI - PMC - PubMed
-
- Izdebska M., Piątkowska-Chmiel I., Korolczuk A., Herbet M., Gawrońska-Grzywacz M., Gieroba R., Sysa M., Czajkowska-Bania K., Cygal M., Korga A., et al. The beneficial effects of resveratrol on the steatosis and mitochondrial oxidative stress in HepG2 cells. Can. J. Physiol. Pharm. 2017;95:1442–1453. doi: 10.1139/cjpp-2016-0561. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

