RBOH-Dependent ROS Synthesis and ROS Scavenging by Plant Specialized Metabolites To Modulate Plant Development and Stress Responses
- PMID: 30781949
- PMCID: PMC6857786
- DOI: 10.1021/acs.chemrestox.9b00028
RBOH-Dependent ROS Synthesis and ROS Scavenging by Plant Specialized Metabolites To Modulate Plant Development and Stress Responses
Abstract
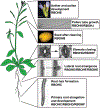
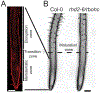
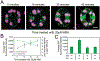
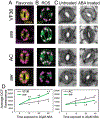
Reactive oxygen species (ROS) regulate plant growth and development. ROS are kept at low levels in cells to prevent oxidative damage, allowing them to be effective signaling molecules upon increased synthesis. In plants and animals, NADPH oxidase/respiratory burst oxidase homolog (RBOH) proteins provide localized ROS bursts to regulate growth, developmental processes, and stress responses. This review details ROS production via RBOH enzymes in the context of plant development and stress responses and defines the locations and tissues in which members of this family function in the model plant Arabidopsis thaliana. To ensure that these ROS signals do not reach damaging levels, plants use an array of antioxidant strategies. In addition to antioxidant machineries similar to those found in animals, plants also have a variety of specialized metabolites that scavenge ROS. These plant specialized metabolites exhibit immense structural diversity and have highly localized accumulation. This makes them important players in plant developmental processes and stress responses that use ROS-dependent signaling mechanisms. This review summarizes the unique properties of plant specialized metabolites, including carotenoids, ascorbate, tocochromanols (vitamin E), and flavonoids, in modulating ROS homeostasis. Flavonols, a subclass of flavonoids with potent antioxidant activity, are induced during stress and development, suggesting that they have a role in maintaining ROS homeostasis. Recent results using genetic approaches have shown how flavonols regulate development and stress responses through their action as antioxidants.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Baxter A, Mittler R, and Suzuki N (2014) ROS as Key Players in Plant Stress Signalling. J. Exp. Bot 65, 1229–1240. - PubMed
-
- Mittler R (2017) ROS Are Good. Trends Plant Sci 22, 11–19. - PubMed
-
- Suzuki N, Miller G, Morales J, Shulaev V, Torres MA, and Mittler R (2011) Respiratory Burst Oxidases: The Engines of ROS Signaling. Curr. Opin. Plant Biol 14, 691–699. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

