Therapeutic blockade of HMGB1 reduces early motor deficits, but not survival in the SOD1G93A mouse model of amyotrophic lateral sclerosis
- PMID: 30782181
- PMCID: PMC6380064
- DOI: 10.1186/s12974-019-1435-2
Therapeutic blockade of HMGB1 reduces early motor deficits, but not survival in the SOD1G93A mouse model of amyotrophic lateral sclerosis
Abstract
Background: Amyotrophic lateral sclerosis (ALS) is a fatal and rapidly progressing neurodegenerative disease without effective treatment. The receptor for advanced glycation end products (RAGE) and the toll-like receptor (TLR) system are major components of the innate immune system, which have been implicated in ALS pathology. Extracellularly released high-mobility group box 1 (HMGB1) is a pleiotropic danger-associated molecular pattern (DAMP), and is an endogenous ligand for both RAGE and TLR4.
Methods: The present study examined the effect of HMGB1 inhibition on disease progression in the preclinical SOD1G93A transgenic mouse model of ALS using a potent anti-HMGB1 antibody (2G7), which targets the extracellular DAMP form of HMGB1.
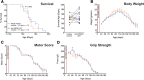
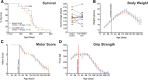
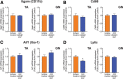
Results: We found that chronic intraperitoneal dosing of the anti-HMGB1 antibody to SOD1G93A mice transiently improved hind-limb grip strength early in the disease, but did not extend survival. Anti-HMGB1 treatment also reduced tumour necrosis factor α and complement C5a receptor 1 gene expression in the spinal cord, but did not affect overall glial activation.
Conclusions: In summary, our results indicate that therapeutic targeting of an extracellular DAMP, HMGB1, improves early motor dysfunction, but overall has limited efficacy in the SOD1G93A mouse model of ALS.
Keywords: Innate immune system; Neuroinflammation; RAGE; TLR4.
Conflict of interest statement
Ethics approval and consent to participate
All experimental procedures were approved by the University of Queensland Animal Ethics Committee and complied with the policies and regulations regarding animal experimentation. They were conducted in accordance with the Queensland Government Animal Research Act 2001, associated Animal Care and Protection Regulations (2002 and 2008) and the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes, 8th Edition (National Health and Medical Research Council, 2013). ARRIVE guidelines have been followed in the preparation of the manuscript.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

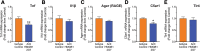

Similar articles
-
Absence of Receptor for Advanced Glycation End Product (RAGE) Reduces Inflammation and Extends Survival in the hSOD1G93A Mouse Model of Amyotrophic Lateral Sclerosis.Mol Neurobiol. 2020 Oct;57(10):4143-4155. doi: 10.1007/s12035-020-02019-9. Epub 2020 Jul 16. Mol Neurobiol. 2020. PMID: 32676989
-
Absence of toll-like receptor 4 (TLR4) extends survival in the hSOD1 G93A mouse model of amyotrophic lateral sclerosis.J Neuroinflammation. 2015 May 13;12:90. doi: 10.1186/s12974-015-0310-z. J Neuroinflammation. 2015. PMID: 25962427 Free PMC article.
-
Toll-Like Receptor-4 Inhibitor TAK-242 Attenuates Motor Dysfunction and Spinal Cord Pathology in an Amyotrophic Lateral Sclerosis Mouse Model.Int J Mol Sci. 2017 Aug 1;18(8):1666. doi: 10.3390/ijms18081666. Int J Mol Sci. 2017. PMID: 28763002 Free PMC article.
-
Implication of HMGB1 signaling pathways in Amyotrophic lateral sclerosis (ALS): From molecular mechanisms to pre-clinical results.Pharmacol Res. 2020 Jun;156:104792. doi: 10.1016/j.phrs.2020.104792. Epub 2020 Apr 8. Pharmacol Res. 2020. PMID: 32278047 Review.
-
The role of high mobility group box 1 in neuroinflammatory related diseases.Biomed Pharmacother. 2023 May;161:114541. doi: 10.1016/j.biopha.2023.114541. Epub 2023 Mar 22. Biomed Pharmacother. 2023. PMID: 36963363 Review.
Cited by
-
Targeting Inflammation Driven by HMGB1.Front Immunol. 2020 Mar 20;11:484. doi: 10.3389/fimmu.2020.00484. eCollection 2020. Front Immunol. 2020. PMID: 32265930 Free PMC article. Review.
-
Novel insights into RAGE signaling pathways during the progression of amyotrophic lateral sclerosis in RAGE-deficient SOD1 G93A mice.PLoS One. 2024 Mar 8;19(3):e0299567. doi: 10.1371/journal.pone.0299567. eCollection 2024. PLoS One. 2024. PMID: 38457412 Free PMC article.
-
The role of immune-mediated alterations and disorders in ALS disease.Hum Immunol. 2021 Mar;82(3):155-161. doi: 10.1016/j.humimm.2021.01.017. Epub 2021 Feb 12. Hum Immunol. 2021. PMID: 33583639 Free PMC article. Review.
-
Desloratadine alleviates ALS-like pathology in hSOD1G93A mice via targeting 5HTR2A on activated spinal astrocytes.Acta Pharmacol Sin. 2024 May;45(5):926-944. doi: 10.1038/s41401-023-01223-2. Epub 2024 Jan 29. Acta Pharmacol Sin. 2024. PMID: 38286832 Free PMC article.
-
Single-cell deconstruction of post-sepsis skeletal muscle and adipose tissue microenvironments.J Cachexia Sarcopenia Muscle. 2020 Oct;11(5):1351-1363. doi: 10.1002/jcsm.12596. Epub 2020 Jul 8. J Cachexia Sarcopenia Muscle. 2020. PMID: 32643301 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

