Melanoma arising in a Giant congenital melanocytic nevus: two case reports
- PMID: 30782194
- PMCID: PMC6381634
- DOI: 10.1186/s13000-019-0797-1
Melanoma arising in a Giant congenital melanocytic nevus: two case reports
Abstract
Background: A giant congenital melanocytic nevus (GCMN) is found in 0.1% of live-born infants. If present, the lesion has a chance of about 6% to develop into malignant melanoma. Both children and adults can be affected by malignant melanoma arising in a giant congenital nevus. Up to 95% of GCMNs harbor NRAS mutations, and mutations in the BRAF, MC1R, TP53, and GNAQ genes have also been described. The individualization of therapy is required, but diagnostic and prognostic criteria remain controversial.
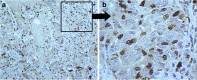
Case presentations: We report two cases: 1) melanoma arising in a giant congenital nevus during the first month of life complicated with neurocutaneous melanosis (NCM), and 2) melanoma arising in a giant congenital nevus during the first 6 months of life. Pathology, immunohistochemistry, and genetic analyses of tumor tissue were performed. The first case revealed only a non-pathogenic P72R polymorphism of the TP53 gene in the homozygote condition. For the second case, a Q61K mutation was detected in the NRAS gene.
Conclusion: Malignant melanoma associated with GCMN is rare and therefore poorly understood. Outcomes have been linked to the stage at diagnosis, but no additional pathological prognostic factors have been identified. The most frequent genetic event in giant CMNs is NRAS mutations, which was discovered in one of our cases. To accumulate evidence to improve disease prognosis and outcomes, children with congenital melanocytic nevus should be included in a systemic follow-up study from birth.
Keywords: Genetic analysis; Giant congenital melanocytic nevus; Infants; Melanoma; NRAS mutation; Neurocutaneous melanosis.
Conflict of interest statement
Ethics approval and consent to participate
Parents of the patients provided informed consent. The study was approved by the Ethical Committee of the Federal State Budgetary Institution “N.N. Blokhin Medical Research Center of Oncology” of the Ministry of Health of the Russian Federation.
Consent for publication
Written informed consents for publication of their clinical details and/or clinical images were obtained from the parents of the patients. A copy of the consent forms is available for review by the Editor of this journal.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





References
-
- Ries L. Cancer Incidence and Survival among children and adolescents: United States SEER Program 1975–1995. Natl Cancer Institute, SEER Progr. 1999.
-
- Paschoal F. Nevo melanocítico congênito. An Bras Dermatol. 2002;77:649–656. doi: 10.1590/S0365-05962002000600002. - DOI
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

