Evolution of our understanding of cell volume regulation by the pump-leak mechanism
- PMID: 30782603
- PMCID: PMC6445581
- DOI: 10.1085/jgp.201812274
Evolution of our understanding of cell volume regulation by the pump-leak mechanism
Erratum in
-
Correction: Evolution of our understanding of cell volume regulation by the pump-leak mechanism.J Gen Physiol. 2019 Apr 1;151(4):606-607. doi: 10.1085/jgp.20181227402282019c. Epub 2019 Mar 6. J Gen Physiol. 2019. PMID: 30842220 Free PMC article. No abstract available.
Abstract
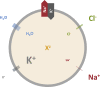
All animal cells are surrounded by a flexible plasma membrane that is permeable to water and to small ions. Cells thus face a fundamental problem: the considerable tension that their membranes would experience if the osmotic influx of water, driven by the presence of impermeant intracellular ions, was left unopposed. The pivotal study that described the cell's remedy for this impending osmotic catastrophe-the "pump-leak mechanism" (PLM)-was published in the Journal of General Physiology by Tosteson and Hoffman in 1960. Their work revealed how the sodium pump stabilizes cell volume by eliminating the osmotic gradient. Here we describe the mechanistic basis of the PLM, trace the history of its discovery, and place it into the context of our current understanding.
© 2019 Kay and Blaustein.
Figures



References
-
- Blaustein M.P.2016. It was the best of times - A postdoctoral experience in the UK in the 1960s. Physiol. News Autumn:32–35.
-
- Blaustein M.P., Kao J.P.Y., and Matteson D.R.. 2019. Cellular Physiology and Neurophysiology. Mosby Physiology Series, third edition Elsevier, St. Louis. In press..
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

