Children with Hemiparesis Arm and Movement Project (CHAMP): protocol for a multisite comparative efficacy trial of paediatric constraint-induced movement therapy (CIMT) testing effects of dosage and type of constraint for children with hemiparetic cerebral palsy
- PMID: 30782701
- PMCID: PMC6340418
- DOI: 10.1136/bmjopen-2018-023285
Children with Hemiparesis Arm and Movement Project (CHAMP): protocol for a multisite comparative efficacy trial of paediatric constraint-induced movement therapy (CIMT) testing effects of dosage and type of constraint for children with hemiparetic cerebral palsy
Abstract
Introduction: The Children with Hemiparesis Arm and Movement Project (CHAMP) addresses two pressing issues concerning paediatric constraint-induced movement therapy (CIMT): effects of two dosages and two types of constraint on functional outcomes. Systematic reviews conclude that CIMT is one of the most efficacious treatments, but wide variations in treatment protocols, outcome measures and patient characteristics have prevented conclusions about potential effects of dosage levels and constraint methods.
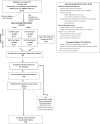
Methods and analysis: CHAMP is a multisite comparative efficacy randomised controlled trial of 135 children (2-8 years) with hemiparetic cerebral palsy. The 2×2 factorial design tests two dosage levels-60 hours (3.0 hours/day, 5 days/week × 4 weeks) and 30 hours (2.5 hours/day, 3 days/week × 4 weeks) and two constraint conditions-full-arm, full-time cast and part-time splint, plus usual and customary (UCT) controls, yielding five groups: (1) 60 hours CIMT+full-time cast, (2) 60 hours CIMT+part-time splint, (3) 30 hours CIMT+full-time cast, (4) 30 hours CIMT+part-time splint and (5) UCT. Trained therapists deliver the standardised ACQUIREc protocol for CIMT. Blinded assessments at baseline, end of treatment, and 6 and 12 months post treatment include the Assisting Hand Assessment, and subscales from the Peabody Developmental Motor Scales-2 and modified Quality of Upper Extremity Skills Test. Parents complete the Pediatric Motor Activity Log and Pediatric Evaluation of Disability Inventory. A new Fidelity of Implementation Rehabilitation Measure monitors treatment delivery. Data analyses involve repeated-measures multivariate analysis of co-variance controlling for selected baseline variables.
Ethics and dissemination: Ethics boards at site universities approved the study protocol. To promote equipoise, parents of UCT controls are offered ACQUIREc after 6 months. A Data Safety and Monitoring Committee reviews results regularly, including measures of child and family stress. We will disseminate CHAMP results via peer-reviewed publications and presentations to professional and advocacy organisations.
Trial registration number: NCT01895660; Pre-results.
Keywords: cerebral palsy; cimt; comparative effectiveness trial; hemiparesis; high intensity treatment; pediatric constraint-induced movement therapy.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures



References
-
- Woodbury ML, Fritz SL, Blanton S, et al. . History and development of CIMT for adults with stroke : Ramey SL, Coker-Bolt P, DeLuca SC, Handbook of Pediatric Constraint-Induced Movement Therapy (CIMT): a guide for occupational therapy and health care clinicians, researchers and educators. Bethesda, MD: AOTA Press, 2013:3–18.
-
- Ramey SL, Coker-Bolt P, DeLuca SC. Handbook of Pediatric Constraint-Induced Movement Therapy (CIMT): a guide for occupational therapy and health care clinicians, researchers and educators. Bethesda, MD: AOTA Press, 2013.
-
- Ramey SL, DeLuca SC, Coker-Bolt P. Operationalizing pediatric CIMT: Guidelines for transforming basic principles and scientific evidence into clinical practice for individual children : Ramey SL, Coker-Bolt P, DeLuca SC, Handbook of Pediatric Constraint-Induced Movement Therapy (CIMT): a guide for occupational therapy and health care clinicians, researchers, and educators. Bethesda, MD: AOTA Press, 2013:115–28.
-
- Ramey SL, DeLuca SC. Appendix A: Key findings from original research articles with functional and occupational outcomes of Pediatric CIMT and related componential interventions : Ramey SL, Coker-Bolt P, DeLuca SC, Handbook of pediatric constraint-induced movement therapy: a guide for occupational therapy and health care clinicians, researchers, and educators. Bethesda, MD: AOTA Press, 2013:283–93.
-
- Ramey SL, DeLuca SC. Research priorities: Understanding and transcending the limits of your current knowledge to inform "best practices" : Ramey SL, Coker-Bolt P, DeLuca SC, Handbook of Pediatric Constraint-Induced Movement Therapy (CIMT): a guide for occupational therapy and health care clinicians, researchers, and educators. Bethesda, MD: AOTA Press, 2013:267–81.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
