Outcomes related to respiratory syncytial virus with an abbreviated palivizumab regimen in children with congenital heart disease: a descriptive analysis
- PMID: 30782771
- PMCID: PMC6380901
- DOI: 10.9778/cmajo.20180167
Outcomes related to respiratory syncytial virus with an abbreviated palivizumab regimen in children with congenital heart disease: a descriptive analysis
Abstract
Background: It has been hypothesized that 4 doses of palivizumab, a neutralizing monoclonal antibody against respiratory syncytial virus (RSV), administered during a fixed-date RSV season may reduce hospital admissions comparably to the standard 5-dose schedule. We report outcomes in children with congenital heart disease approved to receive this 4-dose palivizumab schedule in British Columbia.
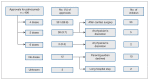
Methods: We performed a population-based descriptive cohort analysis of all 406 approved palivizumab courses over 4 seasons (2012/13 to 2015/16) in 325 children with hemodynamically significant congenital heart disease enrolled in the British Columbia RSV Immunoprophylaxis Program. The primary outcome was in-season hospital admission for potential RSV-related lower respiratory tract infection (LRTI). Secondary outcomes include timing of admission in relation to dosing. Analysis was by intention-to-treat.
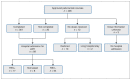
Results: Of the 406 approved palivizumab courses, 391 were administered. In 33 cases (8.4%), an additional dose was given immediately after cardiac bypass surgery. There were 17 RSV-confirmed hospital admissions (median age of children 5.9 mo [interquartile range 4-10 mo]) and 8 admissions in which the child was not tested for RSV, for a maximum of 25 potential RSV-related admissions (6.2 per 100 approvals [95% confidence interval 4.0-9.0]). Twenty-four (96%) of the 25 admissions occurred within the 4-dose palivizumab dosing period, and the remaining admission occurred 52 days after the fourth dose. Sixty-four (72%) of 89 admissions were RSV-negative; the baseline clinical characteristics of these children were not different from those of children with RSV-confirmed admissions.
Interpretation: In infants with hemodynamically significant congenital heart disease, a 4-dose fixed-date palivizumab schedule over a 6-month season provided seasonal protection comparable to that in a clinical trial involving a standard 5-dose schedule. Because RSV was responsible for only 19% of admissions for LRTI in our cohort, it is critical to continue to emphasize other preventive measures, including family education toward proper hand hygiene, breast-feeding and limiting infectious exposures in children at high risk.
Copyright 2019, Joule Inc. or its licensors.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Shi T, McAllister DA, O’Brien KL, et al. RSV Global Epidemiology Network. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390:946–58. - PMC - PubMed
-
- American Academy of Pediatrics Committee on Infectious Diseases; American Academy of Pediatrics Bronchiolitis Guidelines Committee. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection [published erratum in Pediatrics 2014;134:1221] Pediatrics. 2014;134:415–20. - PubMed
-
- Feltes TF, Cabalka AK, Meissner HC, et al. Cardiac Synagis Study Group. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr. 2003;143:532–40. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
