Egg-based influenza split virus vaccine with monoglycosylation induces cross-strain protection against influenza virus infections
- PMID: 30782805
- PMCID: PMC6410868
- DOI: 10.1073/pnas.1819197116
Egg-based influenza split virus vaccine with monoglycosylation induces cross-strain protection against influenza virus infections
Abstract
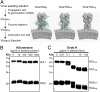
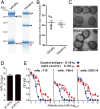
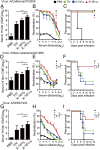
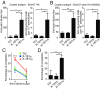
Each year influenza virus infections cause hundreds of thousands of deaths worldwide and a significant level of morbidity with major economic burden. At the present time, vaccination with inactivated virus vaccine produced from embryonated chicken eggs is the most prevalent method to prevent the infections. However, current influenza vaccines are only effective against closely matched circulating strains and must be updated and administered yearly. Therefore, generating a vaccine that can provide broad protection is greatly needed for influenza vaccine development. We have previously shown that vaccination of the major surface glycoprotein hemagglutinin (HA) of influenza virus with a single N-acetylglucosamine at each of the N-glycosylation sites [monoglycosylated HA (HAmg)] can elicit better cross-protection compared with the fully glycosylated HA (HAfg). In the current study, we produced monoglycosylated inactivated split H1N1 virus vaccine from chicken eggs by the N-glycosylation process inhibitor kifunensine and the endoglycosidase Endo H, and intramuscularly immunized mice to examine its efficacy. Compared with vaccination of the traditional influenza vaccine with complex glycosylations from eggs, the monoglycosylated split virus vaccine provided better cross-strain protection against a lethal dose of virus challenge in mice. The enhanced antibody responses induced by the monoglycosylated vaccine immunization include higher neutralization activity, higher hemagglutination inhibition, and more HA stem selectivity, as well as, interestingly, higher antibody-dependent cellular cytotoxicity. This study provides a simple and practical procedure to enhance the cross-strain protection of influenza vaccine by removing the outer part of glycans from the virus surface through modifications of the current egg-based process.
Keywords: N-glycosylation; hemagglutinin stem-specific antibody; influenza vaccines; monoglycosylated influenza split virus vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
An Improved Inactivated Influenza Vaccine with Enhanced Cross Protection.Front Immunol. 2018 Aug 9;9:1815. doi: 10.3389/fimmu.2018.01815. eCollection 2018. Front Immunol. 2018. PMID: 30140267 Free PMC article.
-
Pilot-scale production of inactivated monoglycosylated split H1N1 influenza virus vaccine provides cross-strain protection against influenza viruses.Antiviral Res. 2023 Aug;216:105640. doi: 10.1016/j.antiviral.2023.105640. Epub 2023 May 30. Antiviral Res. 2023. PMID: 37263355
-
Neuraminidase-Inhibiting Antibody Titers Correlate with Protection from Heterologous Influenza Virus Strains of the Same Neuraminidase Subtype.J Virol. 2018 Aug 16;92(17):e01006-18. doi: 10.1128/JVI.01006-18. Print 2018 Sep 1. J Virol. 2018. PMID: 29925654 Free PMC article.
-
Influenza and Antibody-Dependent Cellular Cytotoxicity.Front Immunol. 2019 Jun 25;10:1457. doi: 10.3389/fimmu.2019.01457. eCollection 2019. Front Immunol. 2019. PMID: 31316510 Free PMC article. Review.
-
Targeting Hemagglutinin: Approaches for Broad Protection against the Influenza A Virus.Viruses. 2019 Apr 30;11(5):405. doi: 10.3390/v11050405. Viruses. 2019. PMID: 31052339 Free PMC article. Review.
Cited by
-
Protein engineering strategies for rational immunogen design.NPJ Vaccines. 2021 Dec 17;6(1):154. doi: 10.1038/s41541-021-00417-1. NPJ Vaccines. 2021. PMID: 34921149 Free PMC article. Review.
-
Synthetic Carbohydrate Chemistry and Translational Medicine.J Org Chem. 2020 Dec 18;85(24):15780-15800. doi: 10.1021/acs.joc.0c01834. Epub 2020 Oct 30. J Org Chem. 2020. PMID: 33125238 Free PMC article.
-
A Carbohydrate-Binding Protein from the Edible Lablab Beans Effectively Blocks the Infections of Influenza Viruses and SARS-CoV-2.Cell Rep. 2020 Aug 11;32(6):108016. doi: 10.1016/j.celrep.2020.108016. Epub 2020 Jul 24. Cell Rep. 2020. PMID: 32755598 Free PMC article.
-
Role of N-linked glycosylation in porcine reproductive and respiratory syndrome virus (PRRSV) infection.J Gen Virol. 2024 May;105(5):001994. doi: 10.1099/jgv.0.001994. J Gen Virol. 2024. PMID: 38776134 Free PMC article.
-
Current Approaches and Applications in Avian Genome Editing.Int J Mol Sci. 2020 May 30;21(11):3937. doi: 10.3390/ijms21113937. Int J Mol Sci. 2020. PMID: 32486292 Free PMC article. Review.
References
-
- Krammer F, Palese P. Advances in the development of influenza virus vaccines. Nat Rev Drug Discov. 2015;14:167–182. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

