Dual AAV-mediated gene therapy restores hearing in a DFNB9 mouse model
- PMID: 30782832
- PMCID: PMC6410774
- DOI: 10.1073/pnas.1817537116
Dual AAV-mediated gene therapy restores hearing in a DFNB9 mouse model
Abstract
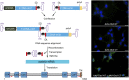
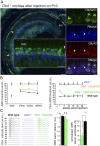
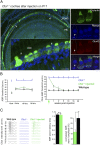
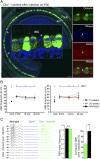
Autosomal recessive genetic forms (DFNB) account for most cases of profound congenital deafness. Adeno-associated virus (AAV)-based gene therapy is a promising therapeutic option, but is limited by a potentially short therapeutic window and the constrained packaging capacity of the vector. We focus here on the otoferlin gene underlying DFNB9, one of the most frequent genetic forms of congenital deafness. We adopted a dual AAV approach using two different recombinant vectors, one containing the 5' and the other the 3' portions of otoferlin cDNA, which exceed the packaging capacity of the AAV when combined. A single delivery of the vector pair into the mature cochlea of Otof-/- mutant mice reconstituted the otoferlin cDNA coding sequence through recombination of the 5' and 3' cDNAs, leading to the durable restoration of otoferlin expression in transduced cells and a reversal of the deafness phenotype, raising hopes for future gene therapy trials in DFNB9 patients.
Keywords: DFNB9; deafness; dual AAV; gene therapy; otoferlin.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
Conflict of interest statement: W.W.H. and the University of Florida (UF) have a financial interest in the use of AAV therapies, and own equity in a company, Applied Genetic Technologies Corp. (AGTC), that might, in the future, commercialize some aspects of this work, and a joint international patent application (International Patent Application No.: PCT/US2018/031009 - WGS “Whole Genome Sequencing” Ref. U1197.70110WO00) has been submitted by UF and University of California, San Francisco (F.D., W.W.H., O.A.).
Figures
References
-
- Kral A, O’Donoghue GM. Profound deafness in childhood. N Engl J Med. 2010;363:1438–1450. - PubMed
-
- Flotte TR, et al. Phase I trial of intramuscular injection of a recombinant adeno-associated virus alpha 1-antitrypsin (rAAV2-CB-hAAT) gene vector to AAT-deficient adults. Hum Gene Ther. 2004;15:93–128. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

