Tramadol/dexketoprofen (TRAM/DKP) compared with tramadol/paracetamol in moderate to severe acute pain: results of a randomised, double-blind, placebo and active-controlled, parallel group trial in the impacted third molar extraction pain model (DAVID study)
- PMID: 30782886
- PMCID: PMC6377526
- DOI: 10.1136/bmjopen-2018-023715
Tramadol/dexketoprofen (TRAM/DKP) compared with tramadol/paracetamol in moderate to severe acute pain: results of a randomised, double-blind, placebo and active-controlled, parallel group trial in the impacted third molar extraction pain model (DAVID study)
Erratum in
-
Correction: Tramadol/dexketoprofen (TRAM/DKP) compared with tramadol/paracetamol in moderate to severe acute pain: results of a randomised, double-blind, placebo and active-controlled, parallel group trial in the impacted third molar extraction pain model (DAVID study).BMJ Open. 2019 Jun 14;9(6):e023715corr1. doi: 10.1136/bmjopen-2018-023715corr1. BMJ Open. 2019. PMID: 31201197 Free PMC article. No abstract available.
Abstract
Objectives: To compare efficacy/safety of oral tramadol 75 mg/dexketoprofen 25 mg (TRAM/DKP) and TRAM 75 mg/paracetamol 650 mg (TRAM/paracetamol) in moderate to severe pain following surgical removal of impacted lower third molar.
Design: Multicentre, randomised, double-blind, placebo-controlled, phase IIIb study.
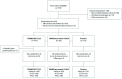
Participants: Healthy adult patients scheduled for surgical extraction of at least one fully/partially impacted lower third molar requiring bone manipulation. 654 patients were randomised and 653 were eligible for analysis.
Interventions: Surgery was performed under local anaesthetic. No sedation was permitted. Patients rated pain intensity (PI) using an 11-Numerical Rating Scale (NRS) (0 no pain; 10 worst pain). Participants experiencing moderate/severe pain (≥4) within 4 hours of surgery were randomised (2:2:1 ratio) to a single oral dose of TRAM/DKP 75/25 mg, TRAM/paracetamol 75/650 mg or placebo.
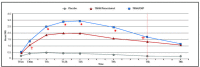
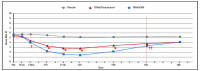
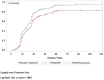
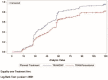
Main outcome measures: Efficacy was based patients' electronic diaries. Analgesia and pain were recorded as follows: pain relief (PAR) on a 5-point Verbal Rating Scale (0='no relief', 1='a little (perceptible) relief', 2='some (meaningful) relief', 3='lot of relief', 4='complete relief') at the predefined postdose time points t15 min, t30 min, t1 hour, t1.5 hour, t2 hour, t4 hour, t6 hour and t8 hour and PI on the 11-point NRS at t0 and at the same predefined postdose time points. Onset of analgesia documented using double stopwatch method over a 2-hour period. Primary endpoint was total pain relief over 6 hours (TOTPAR6). Rescue medication was available during the treatment period.
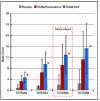
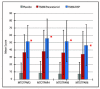
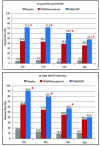
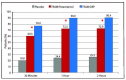
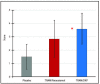
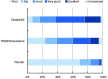
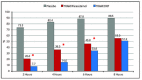
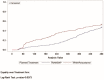
Results: TRAM/DKP was superior to TRAM/paracetamol and placebo at the primary endpoint TOTPAR6 (p<0.0001). Mean (SD) TOTPAR6 in the TRAM/DKP group was 13 (6.97), while those in the active control and placebo groups were 9.2 (7.65) and 1.9 (3.89), respectively. Superiority of TRAM/DKP over active comparator and placebo was observed at all secondary endpoints. Incidence of adverse events was comparable between active groups.
Conclusions: TRAM/DKP (75/25 mg) is effective and superior to TRAM/paracetamol (75/650 mg) in relieving moderate to severe acute pain following surgical removal of impacted lower third molar, with a faster onset of action, greater and durable analgesia, together with a favourable safety profile.
Trial registration number: EudraCT 2015-004152-22 and NCT02777970.
Keywords: clinical trials; oral medicine; pain management; primary care; public health.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: MH reports personal fees as a consultant for educational symposia by Menarini Group. CG-E reports personal fees from Menarini Group, outside the submitted work. TD reports grants from Menarini Group, during the conduct of the study; grants and personal fees from Institut Biochimique SA, outside the submitted work. AM, SM, EG, TBZ and GV declare no conflict of interest. We attest that we have obtained appropriate permissions and paid any required fees for use of copyright protected materials.
Figures
References
-
- Chou R, Gordon DB, de Leon-Casasola OA, et al. . Management of postoperative pain: a clinical practice guideline from the american pain society, the american society of regional anesthesia and pain medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain 2016;17:131–57. 10.1016/j.jpain.2015.12.008 - DOI - PubMed
-
- White PF. Multimodal analgesia: its role in preventing postoperative pain. Curr Opin Investig Drugs 2008;9:76–82. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
