Effectiveness and cost-effectiveness randomised controlled trial of basic versus biofeedback-mediated intensive pelvic floor muscle training for female stress or mixed urinary incontinence: protocol for the OPAL (optimising pelvic floor exercises to achieve long-term benefits) trial mixed methods longitudinal qualitative case study and process evaluation
- PMID: 30782894
- PMCID: PMC6411251
- DOI: 10.1136/bmjopen-2018-024152
Effectiveness and cost-effectiveness randomised controlled trial of basic versus biofeedback-mediated intensive pelvic floor muscle training for female stress or mixed urinary incontinence: protocol for the OPAL (optimising pelvic floor exercises to achieve long-term benefits) trial mixed methods longitudinal qualitative case study and process evaluation
Abstract
Introduction: Female urinary incontinence (UI) is common affecting up to 45% of women. Pelvic floor muscle training (PFMT) is the first-line treatment but there is uncertainty whether intensive PFMT is better than basic PFMT for long-term symptomatic improvement. It is also unclear which factors influence women's ability to perform PFMT long term and whether this has impacts on long-term outcomes. OPAL (optimising PFMT to achieve long-term benefits) trial examines the effectiveness and cost-effectiveness of basic PFMT versus biofeedback-mediated PFMT and this evaluation explores women's experiences of treatment and the factors which influence effectiveness. This will provide data aiding interpretation of the trial findings; make recommendations for optimising the treatment protocol; support implementation in practice; and address gaps in the literature around long-term adherence to PFMT for women with stress or mixed UI.
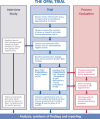
Methods and analysis: This evaluation comprises a longitudinal qualitative case study and process evaluation (PE). The case study aims to explore women's experiences of treatment and adherence and the PE will explore factors influencing intervention effectiveness. The case study has a two-tailed design and will recruit 40 women, 20 from each trial group; they will be interviewed four times over 2 years. Process data will be collected from women through questionnaires at four time-points, from health professionals through checklists and interviews and by sampling 100 audio recordings of appointments. Qualitative analysis will use case study methodology (qualitative study) and the framework technique (PE) and will interrogate for similarities and differences between the trial groups regarding barriers and facilitators to adherence. Process data analyses will examine fidelity, engagement and mediating factors using descriptive and interpretative statistics.
Ethics and dissemination: Approval from West of Scotland Research Ethics Committee 4 (16/LO/0990). Findings will be published in journals, disseminated at conferences and through the final report.
Trial registration number: ISRCTN57746448.
Keywords: biofeedback; comparative case study; pelvic floor muscle training; process evaluation; qualitative; urinary incontinence.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Abrams P, Cardozo L, Wagg A, et al. Incontinence. 6th ed Bristol: Health Publications Ltd, 2017.
-
- Perry S, Shaw C, Assassa P, et al. An epidemiological study to establish the prevalence of urinary symptoms and felt need in the community: the Leicestershire MRC Incontinence Study. Leicestershire MRC Incontinence Study Team. J Public Health Med 2000;22:427–34. 10.1093/pubmed/22.3.427 - DOI - PubMed
-
- NICE. Urinary incontinence in women. Quality standard (QS77): National Institute for Health and Care Excellence, 2015.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical