Effectiveness and cost-effectiveness of basic versus biofeedback-mediated intensive pelvic floor muscle training for female stress or mixed urinary incontinence: protocol for the OPAL randomised trial
- PMID: 30782895
- PMCID: PMC6411252
- DOI: 10.1136/bmjopen-2018-024153
Effectiveness and cost-effectiveness of basic versus biofeedback-mediated intensive pelvic floor muscle training for female stress or mixed urinary incontinence: protocol for the OPAL randomised trial
Abstract
Introduction: Accidental urine leakage is a distressing problem that affects around one in three women. The main types of urinary incontinence (UI) are stress, urgency and mixed, with stress being most common. Current UK guidelines recommend that women with UI are offered at least 3 months of pelvic floor muscle training (PFMT). There is evidence that PFMT is effective in treating UI, however it is not clear how intensively women have to exercise to give the maximum sustained improvement in symptoms, and how we enable women to achieve this. Biofeedback is an adjunct to PFMT that may help women exercise more intensively for longer, and thus may improve continence outcomes when compared with PFMT alone. A Cochrane review was inconclusive about the benefit of biofeedback, indicating the need for further evidence.
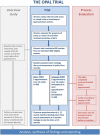
Methods and analysis: This multicentre randomised controlled trial will compare the effectiveness and cost-effectiveness of PFMT versus biofeedback-mediated PFMT for women with stress UI or mixed UI. The primary outcome is UI severity at 24 months after randomisation. The primary economic outcome measure is incremental cost per quality-adjusted life-year at 24 months. Six hundred women from UK community, outpatient and primary care settings will be randomised and followed up via questionnaires, diaries and pelvic floor assessment. All participants are offered six PFMT appointments over 16 weeks. The use of clinic and home biofeedback is added to PFMT for participants in the biofeedback group. Group allocation could not be masked from participants and healthcare staff. An intention-to-treat analysis of the primary outcome will estimate the mean difference between the trial groups at 24 months using a general linear mixed model adjusting for minimisation covariates and other important prognostic covariates, including the baseline score.
Ethics and dissemination: Approval granted by the West of Scotland Research Ethics Committee 4 (16/LO/0990). Written informed consent will be obtained from participants by the local research team. Serious adverse events will be reported to the data monitoring and ethics committee, the ethics committee and trial centres as required. A Standard Protocol Items: Recommendations for Interventional Trials checklist and figure are available for this protocol. The results will be published in international journals and included in the relevant Cochrane review.
Trial registration number: ISRCTN57746448; Pre-results.
Keywords: adherence; biofeedback; electromyography; long-term; pelvic floor muscle training; urinary incontinence.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Abrams P, Cardozo L, Wagg A, et al. . Incontinence. 6th edn: Health Publication Ltd, 2017.
-
- Perry S, Shaw C, Assassa P, et al. . An epidemiological study to establish the prevalence of urinary symptoms and felt need in the community: the Leicestershire MRC Incontinence Study. Leicestershire MRC Incontinence Study Team. J Public Health Med 2000;22:427–34. 10.1093/pubmed/22.3.427 - DOI - PubMed
-
- Melville JL, Fan MY, Rau H, et al. . Major depression and urinary incontinence in women: temporal associations in an epidemiologic sample. Am J Obstet Gynecol 2009;201:490el–490e7. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical