Multicentre randomised controlled trial to investigate usefulness of the rapid diagnostic βLACTA test performed directly on bacterial cell pellets from respiratory, urinary or blood samples for the early de-escalation of carbapenems in septic intensive care unit patients: the BLUE-CarbA protocol
- PMID: 30782909
- PMCID: PMC6367973
- DOI: 10.1136/bmjopen-2018-024561
Multicentre randomised controlled trial to investigate usefulness of the rapid diagnostic βLACTA test performed directly on bacterial cell pellets from respiratory, urinary or blood samples for the early de-escalation of carbapenems in septic intensive care unit patients: the BLUE-CarbA protocol
Abstract
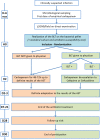
Introduction: The dramatic increase of the incidence of infections caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBL-PE) has led to an increase of 50% of carbapenem consumption all around Europe in only 5 years. This favours the spread of carbapenem-resistant Gram-negative bacilli (GNB), causing life-threatening infections. In order to limit use of carbapenems for infections actually due to ESBL-PE, health authorities promote the use of rapid diagnostic tests of bacterial resistance. The objective of this work conducted in the intensive care unit (ICU) is to determine whether an early de-escalation of empirical carbapenems guided by the result of the βLACTA test is not inferior to the reference strategy of de-escalating carbapenems after the antibiogram result has been rendered.
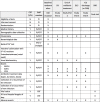
Methods and analysis: This multicentre randomised controlled open-label non-inferiority clinical trial will include patients suffering from respiratory and/or urinary and/or bloodstream infections documented with GNB on direct examination and empirically treated with carbapenems. Empirical carbapenems will be adapted before the second dose depending on the results of the βLACTA test performed directly on the microbiological sample (intervention group) or after 48-72 hours depending on the definite antibiogram (control group). The primary outcome will combine 90-day mortality and percentage of infection recurrence during the ICU stay. The secondary outcomes will include the number of carbapenems defined daily doses and carbapenem-free days after inclusion, the proportion of new infections during ICU stay, new colonisation of patients' digestive tractus with multidrug-resistant GNB, ICU and hospital length of stay and cost-effectiveness ratio.
Ethics and dissemination: This protocol has been approved by the ethics committee of Paris-Ile-de-France IV, and will be carried out according to the principles of the Declaration of Helsinki and the Good Clinical Practice guidelines. The results of this study will be disseminated through presentation at scientific conferences and publication in peer-reviewed journals.
Trial registration number: NCT03147807.
Keywords: antibiotic de-escalation; antibiotic resistance; carbapenem; intensive care unit; rapid diagnostic test; respiratory infection.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- European Center for Disease Control. European Country Overview of Antimicrobial Consumption. 2018. https://www.ecdc.europa.eu/en/antimicrobial-consumption/database/country... (Accessed 9 May 2018).
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical