The Evolution of Azole Resistance in Candida albicans Sterol 14α-Demethylase (CYP51) through Incremental Amino Acid Substitutions
- PMID: 30783005
- PMCID: PMC6496074
- DOI: 10.1128/AAC.02586-18
The Evolution of Azole Resistance in Candida albicans Sterol 14α-Demethylase (CYP51) through Incremental Amino Acid Substitutions
Abstract
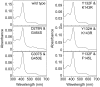
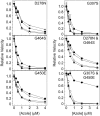
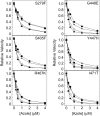
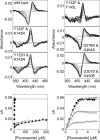
Recombinant Candida albicans CYP51 (CaCYP51) proteins containing 23 single and 5 double amino acid substitutions found in clinical strains and the wild-type enzyme were expressed in Escherichia coli and purified by Ni2+-nitrilotriacetic acid agarose chromatography. Catalytic tolerance to azole antifungals was assessed by determination of the concentration causing 50% enzyme inhibition (IC50) using CYP51 reconstitution assays. The greatest increase in the IC50 compared to that of the wild-type enzyme was observed with the five double substitutions Y132F+K143R (15.3-fold), Y132H+K143R (22.1-fold), Y132F+F145L (10.1-fold), G307S+G450E (13-fold), and D278N+G464S (3.3-fold). The single substitutions K143R, D278N, S279F, S405F, G448E, and G450E conferred at least 2-fold increases in the fluconazole IC50, and the Y132F, F145L, Y257H, Y447H, V456I, G464S, R467K, and I471T substitutions conferred increased residual CYP51 activity at high fluconazole concentrations. In vitro testing of select CaCYP51 mutations in C. albicans showed that the Y132F, Y132H, K143R, F145L, S405F, G448E, G450E, G464S, Y132F+K143R, Y132F+F145L, and D278N+G464S substitutions conferred at least a 2-fold increase in the fluconazole MIC. The catalytic tolerance of the purified proteins to voriconazole, itraconazole, and posaconazole was far lower and limited to increased residual activities at high triazole concentrations for certain mutations rather than large increases in IC50 values. Itraconazole was the most effective at inhibiting CaCYP51. However, when tested against CaCYP51 mutant strains, posaconazole seemed to be the most resistant to changes in MIC as a result of CYP51 mutation compared to itraconazole, voriconazole, or fluconazole.
Keywords: Candida albicans CYP51; azole; mutations.
Copyright © 2019 Warrilow et al.
Figures

References
-
- Magill SS, Swoboda SM, Shields CE, Colantuoni EA, Fothergill AW, Merz WG, Lipsett PA, Hendrix CW. 2009. The epidemiology of Candida colonization and invasive candidiasis in a surgical intensive care unit where fluconazole prophylaxis is utilized: follow-up to a randomized clinical trial. Ann Surg 249:657–665. doi:10.1097/SLA.0b013e31819ed914. - DOI - PubMed
-
- Horn DL, Neofytos D, Anaissie EJ, Fishman JA, Steinbach WJ, Olyaei AJ, Marr KA, Pfaller MA, Chang C-H, Webster KM. 2009. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis 48:1695–1703. doi:10.1086/599039. - DOI - PubMed
-
- Neofytos D, Horn D, Anaissie E, Steinbach W, Olyaei A, Fishman J, Pfaller M, Chang C, Webster K, Marr K. 2009. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis 48:265–273. doi:10.1086/595846. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

