Predicting Short-term MCI-to-AD Progression Using Imaging, CSF, Genetic Factors, Cognitive Resilience, and Demographics
- PMID: 30783207
- PMCID: PMC6381141
- DOI: 10.1038/s41598-019-38793-3
Predicting Short-term MCI-to-AD Progression Using Imaging, CSF, Genetic Factors, Cognitive Resilience, and Demographics
Abstract
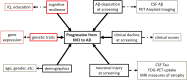
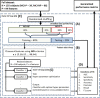
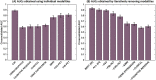
In the Alzheimer's disease (AD) continuum, the prodromal state of mild cognitive impairment (MCI) precedes AD dementia and identifying MCI individuals at risk of progression is important for clinical management. Our goal was to develop generalizable multivariate models that integrate high-dimensional data (multimodal neuroimaging and cerebrospinal fluid biomarkers, genetic factors, and measures of cognitive resilience) for identification of MCI individuals who progress to AD within 3 years. Our main findings were i) we were able to build generalizable models with clinically relevant accuracy (~93%) for identifying MCI individuals who progress to AD within 3 years; ii) markers of AD pathophysiology (amyloid, tau, neuronal injury) accounted for large shares of the variance in predicting progression; iii) our methodology allowed us to discover that expression of CR1 (complement receptor 1), an AD susceptibility gene involved in immune pathways, uniquely added independent predictive value. This work highlights the value of optimized machine learning approaches for analyzing multimodal patient information for making predictive assessments.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Alzheimer’s Association. 2018 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia14(3), 367–429, 10.1016/j.jalz.2018.02.001 (2018). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

