Elevated expression of microRNA-378 in children with asthma aggravates airway remodeling by promoting the proliferation and apoptosis resistance of airway smooth muscle cells
- PMID: 30783418
- PMCID: PMC6364182
- DOI: 10.3892/etm.2018.7141
Elevated expression of microRNA-378 in children with asthma aggravates airway remodeling by promoting the proliferation and apoptosis resistance of airway smooth muscle cells
Abstract
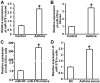
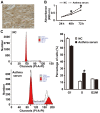
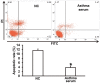
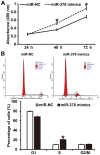
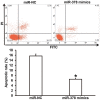
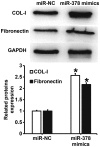
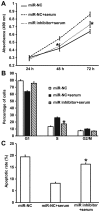
The present study determined the expression of microRNA (miR)-378 in the peripheral blood and lung tissues of children with asthma, and investigated its effect and mechanism of action on the biological functions of airway smooth muscle cells. A total of 23 asthmatic children and 15 healthy children were included in the study. Peripheral blood and tissues were obtained from asthmatic children. Healthy children provided peripheral blood. Quantitative real-time polymerase chain reaction was used to determine the expression of miR-378. Airway smooth muscle cells were isolated and cultured in vitro. The cells were transfected with miR-378 mimics or miR-378 inhibitor. Following transfection, proliferation of the cells was determined using the CCK-8 assay. In addition, flow cytometry was used to detect the cell cycles and apoptosis of smooth muscle cells. Western blotting was performed to determine the expression of extracellular matrix proteins in smooth muscle cells. Furthermore, bioinformatics was used to predict potential target genes of miR-378 and their downstream signaling pathways. Results indicated that the expression of miR-378 in peripheral blood and lung tissues from asthmatic children was increased compared with that in healthy children. Serum from asthmatic children promoted the proliferation of smooth muscle cells in vitro by affecting the cell cycle, and enhanced apoptotic resistance of smooth muscle cells. Notably, overexpression of miR-378 increased the proliferation of smooth muscle cells by affecting the cell cycle, and this upregulated apoptotic resistance of smooth muscle cells and enhanced the expression of extracellular matrix-related proteins in smooth muscle cells. However, downregulation of miR-378 expression reversed the promoting effect of serum from asthmatic children on the biological functions of smooth muscle cells. These findings suggested that miR-378 possibly affects the proliferation, apoptosis and motility of airway smooth muscle cells via downstream signaling pathways. To conclude, the present study demonstrated that miR-378 expression was elevated in the peripheral blood and lung tissues from children with asthma. Furthermore, miR-378 promoted the biological functions of extracellular matrix-related proteins of smooth muscle cells, and possibly exerts its effect via its target genes through downstream signaling pathways.
Keywords: airway smooth muscle cells; asthma; microRNA-378.
Figures
Similar articles
-
MicroRNA-142 Inhibits Proliferation and Promotes Apoptosis in Airway Smooth Muscle Cells During Airway Remodeling in Asthmatic Rats via the Inhibition of TGF-β -Dependent EGFR Signaling Pathway.Cell Physiol Biochem. 2018;47(4):1682-1695. doi: 10.1159/000490986. Epub 2018 Jun 27. Cell Physiol Biochem. 2018. Retraction in: Cell Physiol Biochem. 2021;55(5):659. doi: 10.33594/000000449. PMID: 29949788 Retracted.
-
MiR-204-5p Inhibits Transforming Growth Factor-β1-Induced Proliferation and Extracellular Matrix Production of Airway Smooth Muscle Cells by Regulating Six1 in Asthma.Int Arch Allergy Immunol. 2020;181(4):239-248. doi: 10.1159/000505064. Epub 2020 Jan 17. Int Arch Allergy Immunol. 2020. PMID: 31955160
-
Constitutive high expression of protein arginine methyltransferase 1 in asthmatic airway smooth muscle cells is caused by reduced microRNA-19a expression and leads to enhanced remodeling.J Allergy Clin Immunol. 2017 Aug;140(2):510-524.e3. doi: 10.1016/j.jaci.2016.11.013. Epub 2017 Jan 9. J Allergy Clin Immunol. 2017. PMID: 28081849
-
MicroRNA-638 inhibits human airway smooth muscle cell proliferation and migration through targeting cyclin D1 and NOR1.J Cell Physiol. 2018 Jan;234(1):369-381. doi: 10.1002/jcp.26930. Epub 2018 Aug 4. J Cell Physiol. 2018. PMID: 30076719 Free PMC article. Review.
-
Mechanistic Links Between Obesity and Airway Pathobiology Inform Therapies for Obesity-Related Asthma.Paediatr Drugs. 2023 May;25(3):283-299. doi: 10.1007/s40272-022-00554-7. Epub 2023 Jan 19. Paediatr Drugs. 2023. PMID: 36656428 Free PMC article. Review.
Cited by
-
Specific microRNA Profile Associated with Inflammation and Lipid Metabolism for Stratifying Allergic Asthma Severity.Int J Mol Sci. 2024 Aug 30;25(17):9425. doi: 10.3390/ijms25179425. Int J Mol Sci. 2024. PMID: 39273372 Free PMC article.
-
The Influence of Severity and Disease Duration on TNF Receptors' Redistribution in Asthma and Rheumatoid Arthritis.Cells. 2022 Dec 20;12(1):5. doi: 10.3390/cells12010005. Cells. 2022. PMID: 36611799 Free PMC article.
-
The Role of miRNAs in Extracellular Matrix Repair and Chronic Fibrotic Lung Diseases.Cells. 2021 Jul 6;10(7):1706. doi: 10.3390/cells10071706. Cells. 2021. PMID: 34359876 Free PMC article. Review.
-
Emerging Advances of Non-coding RNAs and Competitive Endogenous RNA Regulatory Networks in Asthma.Bioengineered. 2021 Dec;12(1):7820-7836. doi: 10.1080/21655979.2021.1981796. Bioengineered. 2021. PMID: 34635022 Free PMC article. Review.
-
Targeting Airway Smooth Muscle Hypertrophy in Asthma: An Approach Whose Time Has Come.J Asthma Allergy. 2021 May 25;14:539-556. doi: 10.2147/JAA.S280247. eCollection 2021. J Asthma Allergy. 2021. PMID: 34079293 Free PMC article. Review.
References
-
- Kothari PH, Qiu W, Croteau-Chonka DC, Martinez FD, Liu AH, Lemanske RF, Jr, Ober C, Krishnan JA, Nicolae DL, Barnes KC, et al. Role of local CpG DNA methylation in mediating the 17q21 asthma susceptibility gasdermin B (GSDMB)/ORMDL sphingolipid biosynthesis regulator 3 (ORMDL3) expression quantitative trait locus. J Allergy Clin Immunol. 2018;141:2282–2286.e6. doi: 10.1016/j.jaci.2017.11.057. - DOI - PMC - PubMed
-
- Davies HM. Living with asthma in 19th-century France: The doctor, Armand Trousseau and the patient, Emile Pereire. J Med Biogr 967772017741763. 2018 - PubMed
LinkOut - more resources
Full Text Sources
