E-cadherin regulates biological behaviors of neural stem cells and promotes motor function recovery following spinal cord injury
- PMID: 30783478
- PMCID: PMC6364216
- DOI: 10.3892/etm.2019.7176
E-cadherin regulates biological behaviors of neural stem cells and promotes motor function recovery following spinal cord injury
Abstract
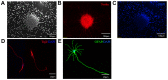
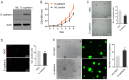
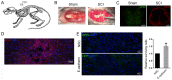
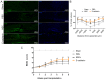
Stem cell-based repair strategies for spinal cord injury (SCI) are a highly studied area of research. Multiple gene-modified stem cells have been transplanted into SCI models, in the hope of generating more neurons to repair a damaged nervous system. However, the results are not always successful, as the grafted cells may be unable to survive in the injured spinal cord. E-cadherin, a transmembrane adhesion protein, has been identified as an epithelial-to-mesenchymal transition marker and is vital for morphological structure maintenance and the functional integrity of epithelial cells. At present, few studies have examined the association between E-cadherin and neural stem cells (NSCs). The present study investigated the expression of E-cadherin in subcultured NSCs and differentiated NSCs. Furthermore, the effect of E-cadherin on NSC viability, migration, differentiation and neurosphere formation was assessed. An in vivo study was used to assess the long-term survival of grafted NSCs. Additionally, the protective effect of E-cadherin on SCI was assessed by analyzing tissue repair, Basso Mouse Scale scores and the expression of inflammatory cytokines. The results of the present study suggested that E-cadherin was able to promote NSC viability and neurosphere formation; however, it had no significant effect on NSC differentiation. To conclude, grafted NSCs with highly expressed E-cadherin facilitated motor function recovery following SCI by reducing the release of inflammatory cytokines.
Keywords: E-cadherin; inflammatory cytokines; neural stem cells; spinal cord injury.
Figures

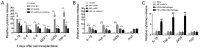
Similar articles
-
Transplantation of Induced Pluripotent Stem Cell-Derived Neural Stem Cells Mediate Functional Recovery Following Thoracic Spinal Cord Injury Through Remyelination of Axons.Stem Cells Transl Med. 2015 Jul;4(7):743-54. doi: 10.5966/sctm.2014-0236. Epub 2015 May 15. Stem Cells Transl Med. 2015. PMID: 25979861 Free PMC article.
-
Anti-Inflammatory Mechanism of Neural Stem Cell Transplantation in Spinal Cord Injury.Int J Mol Sci. 2016 Aug 23;17(9):1380. doi: 10.3390/ijms17091380. Int J Mol Sci. 2016. PMID: 27563878 Free PMC article.
-
LncRNA-GAS5 promotes spinal cord repair and the inhibition of neuronal apoptosis via the transplantation of 3D printed scaffold loaded with induced pluripotent stem cell-derived neural stem cells.Ann Transl Med. 2021 Jun;9(11):931. doi: 10.21037/atm-21-2570. Ann Transl Med. 2021. PMID: 34350246 Free PMC article.
-
[Transplantation of neural stem cells for spinal cord injury].Rinsho Shinkeigaku. 2005 Nov;45(11):874-6. Rinsho Shinkeigaku. 2005. PMID: 16447750 Review. Japanese.
-
The neuronal differentiation microenvironment is essential for spinal cord injury repair.Organogenesis. 2017 Jul 3;13(3):63-70. doi: 10.1080/15476278.2017.1329789. Epub 2017 Jun 9. Organogenesis. 2017. PMID: 28598297 Free PMC article. Review.
Cited by
-
Examining structure-activity relationships of ManNAc analogs used in the metabolic glycoengineering of human neural stem cells.Biomater Adv. 2025 Apr;169:214144. doi: 10.1016/j.bioadv.2024.214144. Epub 2024 Dec 7. Biomater Adv. 2025. PMID: 39754871
-
The potential of gene therapies for spinal cord injury repair: a systematic review and meta-analysis of pre-clinical studies.Neural Regen Res. 2023 Feb;18(2):299-305. doi: 10.4103/1673-5374.347941. Neural Regen Res. 2023. PMID: 35900407 Free PMC article.
-
Whole genome sequencing identifies genetic variants associated with neurogenic inflammation in rosacea.Nat Commun. 2023 Jul 5;14(1):3958. doi: 10.1038/s41467-023-39761-2. Nat Commun. 2023. PMID: 37402769 Free PMC article.
-
Developmental stage of transplanted neural progenitor cells influences anatomical and functional outcomes after spinal cord injury in mice.Commun Biol. 2023 May 19;6(1):544. doi: 10.1038/s42003-023-04893-0. Commun Biol. 2023. PMID: 37208439 Free PMC article.
-
The activation of dormant ependymal cells following spinal cord injury.Stem Cell Res Ther. 2023 Jul 5;14(1):175. doi: 10.1186/s13287-023-03395-4. Stem Cell Res Ther. 2023. PMID: 37408068 Free PMC article. Review.
References
-
- Pomeshchik Y, Puttonen KA, Kidin I, Ruponen M, Lehtonen S, Malm T, Åkesson E, Hovatta O, Koistinaho J. Transplanted human induced pluripotent stem cell-derived neural progenitor cells do not promote functional recovery of pharmacologically immunosuppressed mice with contusion spinal cord injury. Cell Transplant. 2015;24:1799–1812. doi: 10.3727/096368914X684079. - DOI - PubMed
-
- Salewski RP, Mitchell RA, Li L, Shen C, Milekovskaia M, Nagy A, Fehlings MG. Transplantation of induced pluripotent stem cell-derived neural stem cells mediate functional recovery following thoracic spinal cord injury through remyelination of axons. Stem Cells Transl Med. 2015;4:743–754. doi: 10.5966/sctm.2014-0236. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
