Oestrogen inhibits PTPRO to prevent the apoptosis of renal podocytes
- PMID: 30783489
- PMCID: PMC6364249
- DOI: 10.3892/etm.2019.7167
Oestrogen inhibits PTPRO to prevent the apoptosis of renal podocytes
Abstract
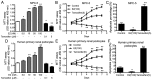
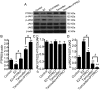
Podocytes are a major component of the glomerular filtration membrane, and their apoptosis is involved in a variety of nephrotic syndromes. In the current study, the effects and molecular mechanisms of oestrogen on the proliferation and apoptosis of podocytes were investigated to elucidate the role of oestrogen in the pathogenesis of childhood nephrotic syndrome. The cell proliferation of mouse renal podocytes (MPC-5) and human primary renal podocytes was promoted by 17β-oestradiol (E2) in what appear to be a time-dependent manner. Apoptosis was inhibited by E2 and promoted by the E2 antagonist, tamoxifen. The expression of protein tyrosine phosphatase receptor type O (PTPRO) decreased with the increasing dosage of E2, but increased with the increasing dosage tamoxifen in MPC-5 and human podocytes. The protein, oestrogen receptor (ER)α, was not expressed in MPC-5 and human podocytes. E2 binding to ERβ completely eliminated PTPRO expression in MPC-5. In podocytes, PTPRO was phosphorylated by E2 at the Y1007 and associated with tyrosine-protein kinase JAK2 (JAK2) activation, rather than JAK1 activation. PTPRO was involved in the binding of E2 to signal transducer and activator of transcription (STAT)3 at the Y705 and S727 sites, resulting in the phosphorylation of STAT3 in podocytes. Through PTPRO, E2 also regulated the proliferation and apoptosis of podocytes. In conclusion, oestrogen binding to ERβ, rather than ERα, promoted the proliferation of podocytes and inhibited the apoptosis of podocytes by inhibiting the expression of PTPRO. The mechanism may be associated with the activation of the JAK2/STAT3 signalling pathway. The current study may provide a novel direction for the treatment of childhood nephrotic syndrome.
Keywords: apoptosis; cell proliferation; oestrogen; protein tyrosine phosphatase receptor type O; renal podocytes.
Figures



References
-
- Welsh GI, Saleem MA. Nephrin-signature molecule of the glomerular podocyte? J Pathol. 2010;220:328–237. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
