An Isoquinoline Scaffold as a Novel Class of Allosteric HIV-1 Integrase Inhibitors
- PMID: 30783506
- PMCID: PMC6378678
- DOI: 10.1021/acsmedchemlett.8b00633
An Isoquinoline Scaffold as a Novel Class of Allosteric HIV-1 Integrase Inhibitors
Abstract
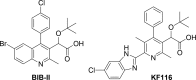
Allosteric HIV-1 integrase inhibitors (ALLINIs) are a new class of potential antiretroviral therapies with a unique mechanism of action and drug resistance profile. To further extend this class of inhibitors via a scaffold hopping approach, we have synthesized a series of analogues possessing an isoquinoline ring system. Lead compound 6l binds in the v-shaped pocket at the IN dimer interface and is highly selective for promoting higher-order multimerization of inactive IN over inhibiting IN-LEDGF/p75 binding. Importantly, 6l potently inhibited HIV-1NL4-3 (A128T IN), which confers marked resistance to archetypal quinoline-based ALLINIs. Thermal degradation studies indicated that at elevated temperatures the acetic acid side chain of specific isoquinoline derivatives undergo decarboxylation reactions. This reactivity has implications for the synthesis of various ALLINI analogues.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
The A128T resistance mutation reveals aberrant protein multimerization as the primary mechanism of action of allosteric HIV-1 integrase inhibitors.J Biol Chem. 2013 May 31;288(22):15813-20. doi: 10.1074/jbc.M112.443390. Epub 2013 Apr 24. J Biol Chem. 2013. PMID: 23615903 Free PMC article.
-
Indole-based allosteric inhibitors of HIV-1 integrase.Bioorg Med Chem Lett. 2016 Oct 1;26(19):4748-4752. doi: 10.1016/j.bmcl.2016.08.037. Epub 2016 Aug 13. Bioorg Med Chem Lett. 2016. PMID: 27568085 Free PMC article.
-
The structural and mechanistic bases for the viral resistance to allosteric HIV-1 integrase inhibitor pirmitegravir.mBio. 2024 Nov 13;15(11):e0046524. doi: 10.1128/mbio.00465-24. Epub 2024 Oct 15. mBio. 2024. PMID: 39404354 Free PMC article.
-
The LEDGF/p75 integrase interaction, a novel target for anti-HIV therapy.Virology. 2013 Jan 5;435(1):102-9. doi: 10.1016/j.virol.2012.09.033. Virology. 2013. PMID: 23217620 Review.
-
Characterization and structural analysis of HIV-1 integrase conservation.AIDS Rev. 2009 Jan-Mar;11(1):17-29. AIDS Rev. 2009. PMID: 19290031 Review.
Cited by
-
Targeting the N-Terminus Domain of the Coronavirus Nucleocapsid Protein Induces Abnormal Oligomerization via Allosteric Modulation.Front Mol Biosci. 2022 Apr 19;9:871499. doi: 10.3389/fmolb.2022.871499. eCollection 2022. Front Mol Biosci. 2022. PMID: 35517857 Free PMC article.
-
HIV-1 integrase binding to genomic RNA 5'-UTR induces local structural changes in vitro and in virio.Retrovirology. 2021 Nov 22;18(1):37. doi: 10.1186/s12977-021-00582-0. Retrovirology. 2021. PMID: 34809662 Free PMC article.
-
Identification and Optimization of a Novel HIV-1 Integrase Inhibitor.ACS Omega. 2022 Jan 24;7(5):4482-4491. doi: 10.1021/acsomega.1c06378. eCollection 2022 Feb 8. ACS Omega. 2022. PMID: 35155940 Free PMC article.
-
Multimodal Functionalities of HIV-1 Integrase.Viruses. 2022 Apr 28;14(5):926. doi: 10.3390/v14050926. Viruses. 2022. PMID: 35632668 Free PMC article. Review.
-
Structural aspects of HIV-1 integrase inhibitors: SAR studies and synthetic strategies.Mol Divers. 2024 Dec 17. doi: 10.1007/s11030-024-11068-4. Online ahead of print. Mol Divers. 2024. PMID: 39690291 Review.
References
-
- Kessl J. J.; Kutluay S. B.; Townsend D.; Rebensburg S.; Slaughter A.; Larue R. C.; Shkriabai N.; Bakouche N.; Fuchs J. R.; Bieniasz P. D.; Kvaratskhelia M. HIV-1 Integrase Binds the Viral RNA Genome and Is Essential during Virion Morphogenesis. Cell 2016, 166, 1257–1268. 10.1016/j.cell.2016.07.044. - DOI - PMC - PubMed
-
- Summa V.; Petrocchi A.; Bonelli F.; Crescenzi B.; Donghi M.; Ferrara M.; Fiore F.; Gardelli C.; Gonzalez Paz O.; Hazuda D. J.; Jones P.; Kinzel O.; Laufer R.; Monteagudo E.; Muraglia E.; Nizi E.; Orvieto F.; Pace P.; Pescatore G.; Scarpelli R.; Stillmock K.; Witmer M. V.; Rowley M. Discovery of Raltegravir, a Potent, Selective Orally Bioavailable HIV-Integrase Inhibitor for the Treatment of HIV-AIDS Infection. J. Med. Chem. 2008, 51, 5843–5855. 10.1021/jm800245z. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

