A Cellular Mechanism Underlying Enhanced Capability for Complex Olfactory Discrimination Learning
- PMID: 30783614
- PMCID: PMC6378325
- DOI: 10.1523/ENEURO.0198-18.2019
A Cellular Mechanism Underlying Enhanced Capability for Complex Olfactory Discrimination Learning
Abstract
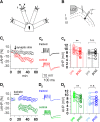
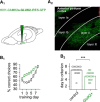
The biological mechanisms underlying complex forms of learning requiring the understanding of rules based on previous experience are not yet known. Previous studies have raised the intriguing possibility that improvement in complex learning tasks requires the long-term modulation of intrinsic neuronal excitability, induced by reducing the conductance of the slow calcium-dependent potassium current (sIAHP) simultaneously in most neurons in the relevant neuronal networks in several key brain areas. Such sIAHP reduction is expressed in attenuation of the postburst afterhyperpolarization (AHP) potential, and thus in enhanced repetitive action potential firing. Using complex olfactory discrimination (OD) learning as a model for complex learning, we show that brief activation of the GluK2 subtype glutamate receptor results in long-lasting enhancement of neuronal excitability in neurons from controls, but not from trained rats. Such an effect can be obtained by a brief tetanic synaptic stimulation or by direct application of kainate, both of which reduce the postburst AHP in pyramidal neurons. Induction of long-lasting enhancement of neuronal excitability is mediated via a metabotropic process that requires PKC and ERK activation. Intrinsic neuronal excitability cannot be modulated by synaptic activation in neurons from GluK2 knock-out mice. Accordingly, these mice are incapable of learning the complex OD task. Moreover, viral-induced overexpression of Gluk2 in piriform cortex pyramidal neurons results in remarkable enhancement of complex OD learning. Thus, signaling via kainate receptors has a central functional role in higher cognitive abilities.
Keywords: GlUk2 receptors; intracellular recordings; meta-learning; olfactory-learning; pyramidal neurons; spost-burst AHP.
Figures


Similar articles
-
Olfactory rule learning-induced enhancement in intrinsic neuronal excitability is maintained by shutdown of the cholinergic M-current.Front Cell Neurosci. 2022 Sep 29;16:934838. doi: 10.3389/fncel.2022.934838. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36246520 Free PMC article.
-
Reversing Aging: Decline in Complex Olfactory Learning Can be Rectified by Restoring Intrinsic Plasticity of Hippocampal CA1 Pyramidal Neurons.Adv Biol (Weinh). 2024 Mar;8(3):e2300323. doi: 10.1002/adbi.202300323. Epub 2023 Dec 25. Adv Biol (Weinh). 2024. PMID: 38145360
-
Persistent ERK activation maintains learning-induced long-lasting modulation of synaptic connectivity.Learn Mem. 2008 Oct 2;15(10):756-61. doi: 10.1101/lm.1127008. Print 2008 Oct. Learn Mem. 2008. PMID: 18832562
-
A non-synaptic mechanism of complex learning: Modulation of intrinsic neuronal excitability.Neurobiol Learn Mem. 2018 Oct;154:30-36. doi: 10.1016/j.nlm.2017.11.015. Epub 2017 Nov 28. Neurobiol Learn Mem. 2018. PMID: 29196146 Review.
-
Cellular correlates of olfactory learning in the rat piriform cortex.Rev Neurosci. 2001;12(2):111-20. doi: 10.1515/revneuro.2001.12.2.111. Rev Neurosci. 2001. PMID: 11392453 Review.
Cited by
-
Association between TRP channels and glutamatergic synapse gene polymorphisms and migraine and the comorbidities anxiety and depression in a Chinese population.Front Genet. 2023 May 26;14:1158028. doi: 10.3389/fgene.2023.1158028. eCollection 2023. Front Genet. 2023. PMID: 37303955 Free PMC article.
-
Olfactory rule learning-induced enhancement in intrinsic neuronal excitability is maintained by shutdown of the cholinergic M-current.Front Cell Neurosci. 2022 Sep 29;16:934838. doi: 10.3389/fncel.2022.934838. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36246520 Free PMC article.
-
Two Signaling Modes Are Better than One: Flux-Independent Signaling by Ionotropic Glutamate Receptors Is Coming of Age.Biomedicines. 2024 Apr 16;12(4):880. doi: 10.3390/biomedicines12040880. Biomedicines. 2024. PMID: 38672234 Free PMC article. Review.
-
Regulation of intrinsic excitability: Roles for learning and memory, aging and Alzheimer's disease, and genetic diversity.Neurobiol Learn Mem. 2019 Oct;164:107069. doi: 10.1016/j.nlm.2019.107069. Epub 2019 Aug 20. Neurobiol Learn Mem. 2019. PMID: 31442579 Free PMC article. Review.
-
The glutamate receptor GluK2 contributes to the regulation of glucose homeostasis and its deterioration during aging.Mol Metab. 2019 Dec;30:152-160. doi: 10.1016/j.molmet.2019.09.011. Epub 2019 Oct 1. Mol Metab. 2019. PMID: 31767166 Free PMC article.
References
-
- Abraham WC, Bear MF (1996) Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci 19:126–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
