Biomimetic potassium-selective nanopores
- PMID: 30783627
- PMCID: PMC6368432
- DOI: 10.1126/sciadv.aav2568
Biomimetic potassium-selective nanopores
Abstract
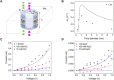
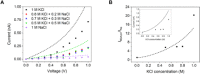
Reproducing the exquisite ion selectivity displayed by biological ion channels in artificial nanopore systems has proven to be one of the most challenging tasks undertaken by the nanopore community, yet a successful achievement of this goal offers immense technological potential. Here, we show a strategy to design solid-state nanopores that selectively transport potassium ions and show negligible conductance for sodium ions. The nanopores contain walls decorated with 4'-aminobenzo-18-crown-6 ether and single-stranded DNA (ssDNA) molecules located at one pore entrance. The ionic selectivity stems from facilitated transport of potassium ions in the pore region containing crown ether, while the highly charged ssDNA plays the role of a cation filter. Achieving potassium selectivity in solid-state nanopores opens new avenues toward advanced separation processes, more efficient biosensing technologies, and novel biomimetic nanopore systems.
Figures


References
-
- Barboiu M., Gilles A., From natural to bioassisted and biomimetic artificial water channel systems. Acc. Chem. Res. 46, 2814–2823 (2013). - PubMed
-
- Hou X., Guo W., Jiang L., Biomimetic smart nanopores and nanochannels. Chem. Soc. Rev. 40, 2385–2401 (2011). - PubMed
-
- Kowalczyk S. W., Blosser T. R., Dekker C., Biomimetic nanopores: Learning from and about nature. Trends Biotechnol. 29, 607–614 (2011). - PubMed
-
- Tagliazucchi M., Szleifer I., Transport mechanisms in nanopores and nanochannels: Can we mimic nature? Mater. Today 18, 131–142 (2015).
-
- B. Hille, Ion Channels of Excitable Membranes (Oxford Univ. Press, 2001).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

