Importance of Prospective Studies in Pregnant and Breastfeeding Women Living With Human Immunodeficiency Virus
- PMID: 30783649
- PMCID: PMC6743813
- DOI: 10.1093/cid/ciz121
Importance of Prospective Studies in Pregnant and Breastfeeding Women Living With Human Immunodeficiency Virus
Abstract
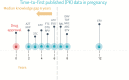
Recently, the US Food and Drug Administration and European Medicines Agency issued warnings on the use of dolutegravir and darunavir/cobicistat for treatment of pregnant women living with human immunodeficiency virus (HIV). It took 3-5 years to identify the risks associated with the use of these antiretroviral drugs, during which time pregnant women were exposed to these drugs in clinical care, outside of controlled clinical trial settings. Across all antiretroviral drugs, the interval between registration of new drugs and first data on pharmacokinetics and safety in pregnancy becoming available is around 6 years. In this viewpoint, we provide considerations for clinical pharmacology research to provide safe and effective treatment of pregnant and breastfeeding women living with HIV and their children. These recommendations will lead to timelier availability of safety and pharmacokinetic information needed to develop safe treatment strategies for pregnant and breastfeeding women living with HIV, and are applicable to other chronic disease areas requiring medication during pregnancy.
Keywords: antiretrovirals; clinical trials; pharmacokinetics; pregnancy; safety.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
References
-
- US Food and Drug Administration. FDA drug safety communication: FDA to evaluate potential risk of neural tube birth defects with HIV medicine dolutegravir (Juluca, Tivicay, Triumeq) 2018. Available at: https://www.fda.gov/drugs/drugsafety/ucm608112.htm. Accessed 10 January 2019.
-
- European Medicines Agency. Safety warning: new study suggests risk of birth defects in babies born to women on HIV medicine dolutegravir 2018. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2018/.... Accessed 10 January 2019.
-
- The Lancet. Dolutegravir for HIV: a lesson in pregnancy safety research. Lancet 2018; 391:2296. - PubMed
-
- World Health Organization. Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV: interim guidance 2018. Available at: http://www.who.int/hiv/pub/guidelines/ARV2018update/en/. Accessed 10 January 2019.
-
- European Medicines Agency. Updated SPC: Rezolsta, UK 2018. Available at: https://www.medicines.org.uk/emc/product/3466/smpc. Accessed 10 January 2019.

