Magnitude and Functionality of the NS1-Specific Antibody Response Elicited by a Live-Attenuated Tetravalent Dengue Vaccine Candidate
- PMID: 30783676
- PMCID: PMC7325620
- DOI: 10.1093/infdis/jiz081
Magnitude and Functionality of the NS1-Specific Antibody Response Elicited by a Live-Attenuated Tetravalent Dengue Vaccine Candidate
Abstract
Background: Dengue virus (DENV) can cause life-threatening disease characterized by endothelial dysfunction and vascular leakage. DENV nonstructural protein 1 (NS1) induces human endothelial hyperpermeability and vascular leak in mice, and NS1 vaccination confers antibody-mediated protective immunity. We evaluated the magnitude, cross-reactivity, and functionality of NS1-specific IgG antibody responses in sera from a phase 2 clinical trial of Takeda's live-attenuated tetravalent dengue vaccine candidate (TAK-003).
Methods: We developed an enzyme-linked immunosorbent assay to measure anti-DENV NS1 IgG in sera from DENV-naive or preimmune subjects pre- and postvaccination with TAK-003 and evaluated the functionality of this response using in vitro models of endothelial permeability.
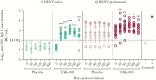
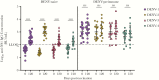
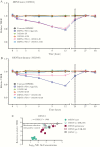
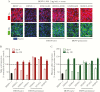
Results: TAK-003 significantly increased DENV-2 NS1-specific IgG in naive individuals, which cross-reacted with DENV-1, -3, and -4 NS1 to varying extents. NS1-induced endothelial hyperpermeability was unaffected by prevaccination serum from naive subjects but was variably inhibited by serum from preimmune subjects. After TAK-003 vaccination, all samples from naive and preimmune vaccinees completely abrogated DENV-2 NS1-induced hyperpermeability and cross-inhibited hyperpermeability induced by DENV-1, -3, and -4 NS1. Inhibition of NS1-induced hyperpermeability correlated with NS1-specific IgG concentrations. Postvaccination sera also prevented NS1-induced degradation of endothelial glycocalyx components.
Conclusion: We provide evidence for functional NS1-specific IgG responses elicited by a candidate dengue vaccine.
Clinical trials registration: NCT01511250.
Keywords: Dengue virus; IgG response; nonstructural protein 1; vaccine.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures


Comment in
-
NS1, Dengue's Dagger.J Infect Dis. 2020 Mar 2;221(6):857-860. doi: 10.1093/infdis/jiz083. J Infect Dis. 2020. PMID: 30783665 No abstract available.
References
-
- World Health Organization. Dengue: guidelines for diagnosis, treatment, prevention, and control. Geneva: World Health Organization, 2009. - PubMed
-
- Capeding MR, Tran NH, Hadinegoro SR, et al. ; CYD14 Study Group Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet 2014; 384:1358–65. - PubMed
-
- Hadinegoro SR, Arredondo-García JL, Capeding MR, et al. ; CYD-TDV Dengue Vaccine Working Group Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med 2015; 373:1195–206. - PubMed
-
- Villar L, Dayan GH, Arredondo-García JL, et al. ; CYD15 Study Group Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med 2015; 372:113–23. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

