Long-term fostamatinib treatment of adults with immune thrombocytopenia during the phase 3 clinical trial program
- PMID: 30784097
- PMCID: PMC6594140
- DOI: 10.1002/ajh.25444
Long-term fostamatinib treatment of adults with immune thrombocytopenia during the phase 3 clinical trial program
Abstract
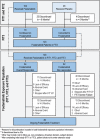
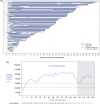
Two randomized, double-blind, placebo-controlled studies demonstrated responses (≥50 000/μL) to fostamatinib in adults with long-standing immune thrombocytopenia (ITP). The long-term safety and efficacy of fostamatinib were evaluated in a follow-on, open-label extension (OLE) study. Patients received double-blind fostamatinib in the randomized trials, and responders continued the same dose, 100 to 150 mg BID, in the OLE study. Nonresponders received 100 mg BID for 4 weeks and could escalate to 150 mg BID at week 4. Endpoints included stable response, platelet count ≥50 000/μL at 4/6 biweekly (randomized trials) or 2/3 monthly visits (OLE), and overall response, ≥1 platelet count ≥50 000/μL during weeks 1 to 12. A total of 146 patients received fostamatinib including 123 in the OLE study. Median treatment duration was 6.7 months. Baseline median ITP duration was 8 years and median platelet count was 16 000/μL; prior treatments included thrombopoietic (TPO) agents (47%), splenectomy (35%), and rituximab (32%). Twenty-seven (18%) patients achieved a stable response with median duration of >28 months and a median platelet count of 89 000/μL. Sixty-four (44%) patients achieved an overall response (including stable responders) with a median platelet count of 63 000/μL and a median response duration of >28 months. Twenty-four of 71 (34%) patients who had failed TPO agents achieved overall responses to fostamatinib. The most common adverse events (AEs) were diarrhea, hypertension, nausea, epistaxis, and abnormal liver function tests. Most AEs were mild/moderate and resolved or were managed with dose reduction, dose interruption, and/or secondary medication. Almost half of the patients achieved an overall response, and most of these maintained their responses for >2 years. No new or increased frequency of AEs was seen at up to 31 months of treatment.
© 2019 The Authors. American Journal of Hematology published by Wiley Periodicals, Inc.
Conflict of interest statement
JB has received research support from Rigel, Novartis, and Amgen, and has participated on advisory boards for Rigel, Novartis, Amgen, Momenta, and Protalex. DMA has received research support from Novartis, Amgen, and Bristol Meyers Squibb, and has been a consultant for Rigel, Amgen, Novartis, and UCB. MB has participated in speakers' bureaus for Rigel, Incyte, and Abbvie. NC has participated in a consultancy or advisory committee for Amgen and Novartis. JM has received research funding from Rigel. HZ, ST and AMD are employees of Rigel Pharmaceuticals.
Figures
References
-
- Frederiksen H, Schmidt K. The incidence of idiopathic thrombocytopenic purpura in adults increases with age. Blood. 1999;94:909‐913. - PubMed
-
- Terrell DR, Beebe LA, Vesely SK, Neas BR, Segal JB, George JN. The incidence of immune thrombocytopenic purpura in children and adults: a critical review of published reports. Am J Hematol. 2010;85:174‐180. - PubMed
-
- Segal JB, Powe NR. Prevalence of immune thrombocytopenia: analyses of administrative data. J Thromb Haemost. 2006;4:2377‐2383. - PubMed
-
- Michel M. Immune thrombocytopenic purpura: epidemiology and implications for patients. Eur J Haematol Suppl. 2009;71:3‐7. - PubMed
-
- Cohen YC, Djulbegovic B, Shamai‐Lubovitz O, Mozes B. The bleeding risk and natural history of idiopathic thrombocytopenic purpura in patients with persistent low platelet counts. Arch Intern Med. 2000;160:1630‐1638. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
