Binding characterization of N-(2-chloro-5-thiomethylphenyl)-N'-(3-[3 H]3 methoxy phenyl)-N'-methylguanidine ([3 H]GMOM), a non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist
- PMID: 30784206
- PMCID: PMC6381215
- DOI: 10.1002/prp2.458
Binding characterization of N-(2-chloro-5-thiomethylphenyl)-N'-(3-[3 H]3 methoxy phenyl)-N'-methylguanidine ([3 H]GMOM), a non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist
Abstract
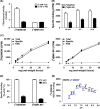
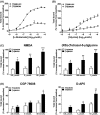
Labeled with carbon-11, N-(2-chloro-5-thiomethylphenyl)-N'-(3-methoxyphenyl)-N'-methylguanidine ([11 C]GMOM) is currently the only positron emission tomography (PET) tracer that has shown selectivity for the ion-channel site of N-methyl-D-aspartate (NMDA) receptors in human imaging studies. The present study reports on the selectivity profile and in vitro binding properties of GMOM. The compound was screened on a panel of 80 targets, and labeled with tritium ([3 H]GMOM). The binding properties of [3 H]GMOM were compared to those of the reference ion-channel ligand [3 H](+)-dizocilpine maleate ([3 H]MK-801), in a set of concentration-response, homologous and heterologous inhibition, and association kinetics assays, performed with repeatedly washed rat forebrain preparations. GMOM was at least 70-fold more selective for NMDA receptors compared to all other targets examined. In homologous inhibition and concentration-response assays, the binding of [3 H]GMOM was regulated by NMDA receptor agonists, albeit in a less prominent manner compared to [3 H]MK-801. Scatchard transformation of homologous inhibition data produced concave upward curves for [3 H]GMOM and [3 H]MK-801. The radioligands showed bi-exponential association kinetics in the presence of 100 μmol L-1 l-glutamate/30 μmol L-1 glycine. [3 H]GMOM (3 nmol L-1 and 10 nmol L-1 ) was inhibited with dual affinity by (+)-MK-801, (R,S)-ketamine and memantine, in both presence and absence of agonists. [3 H]MK-801 (2 nmol L-1 ) was inhibited in a monophasic manner by GMOM under baseline and combined agonist conditions, with an IC50 value of ~19 nmol L-1 . The non-linear Scatchard plots, biphasic inhibition by open channel blockers, and bi-exponential kinetics of [3 H]GMOM indicate a complex mechanism of interaction with the NMDA receptor ionophore. The implications for quantifying the PET signal of [11 C]GMOM are discussed.
Keywords: N; NMDA receptor; N′-diaryl-N-methylguanidine; [3H]GMOM; [3H]MK-801; binding; ion-channel.
© 2019 The Authors. Pharmacology Research & Perspectives published by John Wiley & Sons Ltd, British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics.
Figures

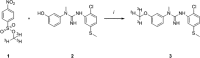




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

