Phase I study of PF‐04895162, a Kv7 channel opener, reveals unexpected hepatotoxicity in healthy subjects, but not rats or monkeys: clinical evidence of disrupted bile acid homeostasis
- PMID: 30784208
- PMCID: PMC6370995
- DOI: 10.1002/prp2.467
Phase I study of PF‐04895162, a Kv7 channel opener, reveals unexpected hepatotoxicity in healthy subjects, but not rats or monkeys: clinical evidence of disrupted bile acid homeostasis
Abstract
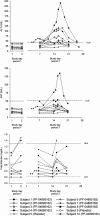
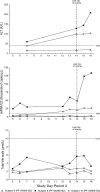
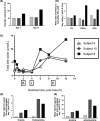
During a randomized Phase 1 clinical trial the drug candidate, PF-04895162 (ICA-105665), caused transaminase elevations (≥grade 1) in six of eight healthy subjects treated at 300 mg twice daily for 2-weeks (NCT01691274). This was unexpected since studies in rats (<6 months) and cynomolgus monkeys (<9 months) treated up to 100 mg/kg/day did not identify the liver as a target organ. Mechanistic studies showed PF-04895162 had low cytotoxic potential in human hepatocytes, but inhibited liver mitochondrial function and bile salt export protein (BSEP) transport. Clinical relevance of these postulated mechanisms of liver injury was explored in three treated subjects that consented to analysis of residual pharmacokinetic plasma samples. Compared to a nonresponder, two subjects with transaminase elevations displayed higher levels of miRNA122 and total/conjugated bile acid species, whereas one demonstrated impaired postprandial clearance of systemic bile acids. Elevated taurine and glycine conjugated to unconjugated bile acid ratios were observed in two subjects, one before the onset of elevated transaminases. Based on the affinity of conjugated bile acid species for transport by BSEP, the profile of plasma conjugated/unconjugated bile acid species was consistent with inhibition of BSEP. These data collectively suggest that the human liver injury by PF-04895162 was due to alterations in bile acid handling driven by dual BSEP/mitochondrial inhibition, two important risk factors associated with drug-induced liver injury in humans. Alterations in systemic bile acid composition were more important than total bile acids in the manifestation of clinical liver injury and may be a very early biomarker of BSEP inhibition.
Keywords: BSEP inhibition; PF-04895162 (ICA-105665); bile acid conjugation status; bile acid homeostasis; hepatotoxicity.
© 2019 The Authors. Pharmacology Research & Perspectives published by John Wiley & Sons Ltd, British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics.
Figures

References
-
- Chalasani NP, Hayashi PH, Bonkovsky HL, Navarro VJ, Lee WM, Fontana RJ and Practice Parameters Committee of the American College of G . ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug‐induced liver injury. Am J Gastroenterol 2014;950‐966; quiz 967. - PubMed
-
- Aleo MD, Luo Y, Swiss R, Bonin PD, Potter DM, Will Y. Human drug‐induced liver injury severity is highly associated with dual inhibition of liver mitochondrial function and bile salt export pump. Hepatology. 2014;1015‐1022. - PubMed
-
- Shah F, Leung L, Barton HA, et al. Setting clinical exposure levels of concern for drug‐induced liver injury (DILI) using mechanistic in vitro assays. Toxicol Sci. 2015;500‐514. - PubMed
-
- Thompson RA, Isin EM, Li Y, et al. In vitro approach to assess the potential for risk of idiosyncratic adverse reactions caused by candidate drugs. Chem Res Toxicol. 2012;1616‐1632. - PubMed
-
- Dykens JA, Will Y. The significance of mitochondrial toxicity testing in drug development. Drug Discov Today. 2007;777‐785. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

