Triphenylphosphonium-Derived Protein Sulfenic Acid Trapping Agents: Synthesis, Reactivity, and Effect on Mitochondrial Function
- PMID: 30784263
- PMCID: PMC6719313
- DOI: 10.1021/acs.chemrestox.8b00385
Triphenylphosphonium-Derived Protein Sulfenic Acid Trapping Agents: Synthesis, Reactivity, and Effect on Mitochondrial Function
Abstract
Redox-mediated protein modifications control numerous processes in both normal and disease metabolism. Protein sulfenic acids, formed from the oxidation of protein cysteine residues, play a critical role in thiol-based redox signaling. The reactivity of protein sulfenic acids requires their identification through chemical trapping, and this paper describes the use of the triphenylphosphonium (TPP) ion to direct known sulfenic acid traps to the mitochondria, a verified source of cellular reactive oxygen species. Coupling of the TPP group with the 2,4-(dioxocyclohexyl)propoxy (DCP) unit and the bicyclo[6.1.0]nonyne (BCN) group produces two new probes, DCP-TPP and BCN-TPP. DCP-TPP and BCN-TPP react with C165A AhpC-SOH, a model protein sulfenic acid, to form the expected adducts with second-order rate constants of k = 1.1 M-1 s-1 and k = 5.99 M-1 s-1, respectively, as determined by electrospray ionization time-of-flight mass spectrometry. The TPP group does not alter the rate of DCP-TPP reaction with protein sulfenic acid compared to dimedone but slows the rate of BCN-TPP reaction compared to a non-TPP-containing BCN-OH control by 4.6-fold. The hydrophobic TPP group may interact with the protein, preventing an optimal reaction orientation for BCN-TPP. Unlike BCN-OH, BCN-TPP does not react with the protein persulfide, C165A AhpC-SSH. Extracellular flux measurements using A549 cells show that DCP-TPP and BCN-TPP influence mitochondrial energetics, with BCN-TPP producing a drastic decrease in basal respiration, perhaps due to its faster reaction kinetics with sulfenylated proteins. Further control experiments with BCN-OH, TPP-COOH, and dimedone provide strong evidence for mitochondrial localization and accumulation of DCP-TPP and BCN-TPP. These results reveal the compatibility of the TPP group with reactive sulfenic acid probes as a mitochondrial director and support the use of the TPP group in the design of sulfenic acid traps.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
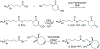




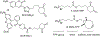
References
-
- Klomsiri C, Nelson KJ, Bechtold E, Soito L, Johnson LC, Lowther WT, Ryu SE, King SB, Furdui CM, and Poole LB (2010) Use of dimedone-based chemical probes for sulfenic acid detection evaluation of conditions affecting probe incorporation into redox-sensitive proteins. Methods Enzymol 473, 77–94. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

