Cellular Compartmentation and the Redox/Nonredox Functions of NAD
- PMID: 30784294
- PMCID: PMC6657305
- DOI: 10.1089/ars.2018.7722
Cellular Compartmentation and the Redox/Nonredox Functions of NAD
Abstract
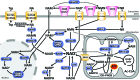
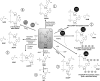
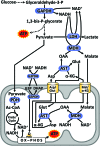
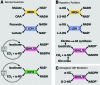
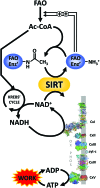
Significance: Nicotinamide adenine dinucleotide (NAD+) spans diverse roles in biology, serving as both an important redox cofactor in metabolism and a substrate for signaling enzymes that regulate protein post-translational modifications (PTMs). Critical Issues: Although the interactions between these different roles of NAD+ (and its reduced form NADH) have been considered, little attention has been paid to the role of compartmentation in these processes. Specifically, the role of NAD+ in metabolism is compartment specific (e.g., mitochondrial vs. cytosolic), affording a very different redox landscape for PTM-modulating enzymes such as sirtuins and poly(ADP-ribose) polymerases in different cell compartments. In addition, the orders of magnitude differences in expression levels between NAD+-dependent enzymes are often not considered when assuming the effects of bulk changes in NAD+ levels on their relative activities. Recent Advances: In this review, we discuss the metabolic, nonmetabolic, redox, and enzyme substrate roles of cellular NAD+, and the recent discoveries regarding the interplay between these roles in different cell compartments. Future Directions: Therapeutic implications for the compartmentation and manipulation of NAD+ biology are discussed. Antioxid. Redox Signal. 31, 623-642.
Keywords: compartmentation; glycolysis; metabolism; mitochondria; nicotinamide adenine dinucleotide; redox.
Figures

Similar articles
-
NAD(H) and NADP(H) Redox Couples and Cellular Energy Metabolism.Antioxid Redox Signal. 2018 Jan 20;28(3):251-272. doi: 10.1089/ars.2017.7216. Epub 2017 Jul 28. Antioxid Redox Signal. 2018. PMID: 28648096 Free PMC article. Review.
-
Interplay between compartmentalized NAD+ synthesis and consumption: a focus on the PARP family.Genes Dev. 2020 Mar 1;34(5-6):254-262. doi: 10.1101/gad.335109.119. Epub 2020 Feb 6. Genes Dev. 2020. PMID: 32029457 Free PMC article. Review.
-
Nicotinamide adenine dinucleotide (NAD+): essential redox metabolite, co-substrate and an anti-cancer and anti-ageing therapeutic target.Biochem Soc Trans. 2020 Jun 30;48(3):733-744. doi: 10.1042/BST20190033. Biochem Soc Trans. 2020. PMID: 32573651 Review.
-
Nicotinamide Adenine Dinucleotide (NAD) Metabolism as a Relevant Target in Cancer.Cells. 2022 Aug 24;11(17):2627. doi: 10.3390/cells11172627. Cells. 2022. PMID: 36078035 Free PMC article. Review.
-
Adipose tissue NAD+-homeostasis, sirtuins and poly(ADP-ribose) polymerases -important players in mitochondrial metabolism and metabolic health.Redox Biol. 2017 Aug;12:246-263. doi: 10.1016/j.redox.2017.02.011. Epub 2017 Feb 27. Redox Biol. 2017. PMID: 28279944 Free PMC article. Review.
Cited by
-
NAD+ metabolism, stemness, the immune response, and cancer.Signal Transduct Target Ther. 2021 Jan 1;6(1):2. doi: 10.1038/s41392-020-00354-w. Signal Transduct Target Ther. 2021. PMID: 33384409 Free PMC article. Review.
-
Location, Location, Location: Compartmentalization of NAD+ Synthesis and Functions in Mammalian Cells.Trends Biochem Sci. 2020 Oct;45(10):858-873. doi: 10.1016/j.tibs.2020.05.010. Epub 2020 Jun 25. Trends Biochem Sci. 2020. PMID: 32595066 Free PMC article. Review.
-
Untargeted Metabolomics Studies of H9c2 Cardiac Cells Submitted to Oxidative Stress, β-Adrenergic Stimulation and Doxorubicin Treatment: Investigation of Cardiac Biomarkers.Front Mol Biosci. 2022 Jun 29;9:898742. doi: 10.3389/fmolb.2022.898742. eCollection 2022. Front Mol Biosci. 2022. PMID: 35847971 Free PMC article.
-
Nicotinamide: Oversight of Metabolic Dysfunction Through SIRT1, mTOR, and Clock Genes.Curr Neurovasc Res. 2020;17(5):765-783. doi: 10.2174/1567202617999201111195232. Curr Neurovasc Res. 2020. PMID: 33183203 Free PMC article. Review.
-
Cardiac metabolism as a driver and therapeutic target of myocardial infarction.J Cell Mol Med. 2020 Jun;24(11):5937-5954. doi: 10.1111/jcmm.15180. Epub 2020 May 8. J Cell Mol Med. 2020. PMID: 32384583 Free PMC article. Review.
References
-
- Aksoy P, White TA, Thompson M, and Chini EN. Regulation of intracellular levels of NAD: a novel role for CD38. Biochem Biophys Res Commun 345: 1386–1392, 2006 - PubMed
-
- Ali HR, Assiri MA, Harris PS, Michel CR, Yun Y, Marentette JO, Huynh FK, Orlicky DJ, Shearn CT, Saba LM, Reisdorph R, Reisdorph N, Hirschey MD, and Fritz KS. Quantifying competition among mitochondrial protein acylation events induced by ethanol metabolism. J Proteome Res 2019. [Epub ahead of print]; DOI: 10.1021/acs.jproteome.8b00800 - DOI - PMC - PubMed
-
- Bai P. Biology of poly(ADP-Ribose) polymerases: the factotums of cell maintenance. Mol Cell 58: 947–958, 2015 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

