An Acinetobacter baumannii, Zinc-Regulated Peptidase Maintains Cell Wall Integrity during Immune-Mediated Nutrient Sequestration
- PMID: 30784584
- PMCID: PMC6441547
- DOI: 10.1016/j.celrep.2019.01.089
An Acinetobacter baumannii, Zinc-Regulated Peptidase Maintains Cell Wall Integrity during Immune-Mediated Nutrient Sequestration
Abstract
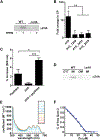
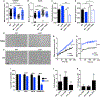
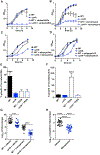
Acinetobacter baumannii is an important nosocomial pathogen capable of causing wound infections, pneumonia, and bacteremia. During infection, A. baumannii must acquire Zn to survive and colonize the host. Vertebrates have evolved mechanisms to sequester Zn from invading pathogens by a process termed nutritional immunity. One of the most upregulated genes during Zn starvation encodes a putative cell wall-modifying enzyme which we named ZrlA. We found that inactivation of zrlA diminished growth of A. baumannii during Zn starvation. Additionally, this mutant strain displays increased cell envelope permeability, decreased membrane barrier function, and aberrant peptidoglycan muropeptide abundances. This altered envelope increases antibiotic efficacy both in vitro and in an animal model of A. baumannii pneumonia. These results establish ZrlA as a crucial link between nutrient metal uptake and cell envelope homeostasis during A. baumannii pathogenesis, which could be targeted for therapeutic development.
Keywords: Acinetobacter; antibiotics; calprotectin; infection; metals; nutritional immunity; peptidase; peptidoglycan; zinc.
Copyright © 2019 Vanderbilt University Medical Center. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures

References
-
- Ammendola S, Pasquali P, Pistoia C, Petrucci P, Petrarca P, Rotilio G, and Battistoni A (2007). High-affinity Zn2+ uptake system ZnuABC is required for bacterial zinc homeostasis in intracellular environments and contributes to the virulence of Salmonella enterica. Infect. Immun 75, 5867–5876. - PMC - PubMed
-
- Antunes LC, Visca P, and Towner KJ (2014). Acinetobacter baumannii: evolution of a global pathogen. Pathog. Dis 71, 292–301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

