Dynamics of the Eukaryotic Replicative Helicase at Lagging-Strand Protein Barriers Support the Steric Exclusion Model
- PMID: 30784593
- PMCID: PMC6381796
- DOI: 10.1016/j.celrep.2019.01.086
Dynamics of the Eukaryotic Replicative Helicase at Lagging-Strand Protein Barriers Support the Steric Exclusion Model
Abstract
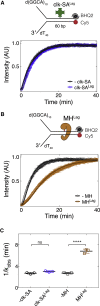
Progression of DNA replication depends on the ability of the replisome complex to overcome nucleoprotein barriers. During eukaryotic replication, the CMG helicase translocates along the leading-strand template and unwinds the DNA double helix. While proteins bound to the leading-strand template efficiently block the helicase, the impact of lagging-strand protein obstacles on helicase translocation and replisome progression remains controversial. Here, we show that CMG and replisome progressions are impaired when proteins crosslinked to the lagging-strand template enhance the stability of duplex DNA. In contrast, proteins that exclusively interact with the lagging-strand template influence neither the translocation of isolated CMG nor replisome progression in Xenopus egg extracts. Our data imply that CMG completely excludes the lagging-strand template from the helicase central channel while unwinding DNA at the replication fork, which clarifies how two CMG helicases could freely cross one another during replication initiation and termination.
Keywords: CMG; DNA-protein crosslink; eukaryotic DNA replication; replicative helicase; single-molecule imaging; steric exclusion.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures






References
-
- Blow J.J., Laskey R.A. A role for the nuclear envelope in controlling DNA replication within the cell cycle. Nature. 1988;332:546–548. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

