Chemical synthesis, crystal structure, versatile evaluation of their biological activities and molecular simulations of novel pyrithiobac derivatives
- PMID: 30784880
- PMCID: PMC7111276
- DOI: 10.1016/j.ejmech.2019.02.002
Chemical synthesis, crystal structure, versatile evaluation of their biological activities and molecular simulations of novel pyrithiobac derivatives
Abstract
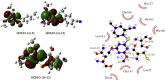
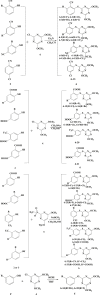
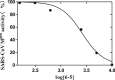
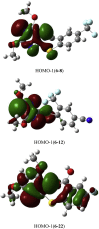
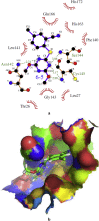
Since pyrithiobac (PTB) is a successful commercial herbicide with very low toxicity against mammals, it is worth exploring its derivatives for an extensive study. Herein, a total of 35 novel compounds were chemically synthesized and single crystal of 6-6 was obtained to confirm the molecular structure of this family of compounds. The novel PTB derivatives were fully evaluated against various biological platforms. From the bioassay results, the best AHAS inhibitor 6-22 displayed weaker herbicidal activity but stronger anti-Candida activity than PTB did. For plant pathogenic fungi, 6-26 showed excellent activity at 50 mg/L dosage. Preliminary insecticidal activity and antiviral activity were also observed for some title compounds. Strikingly, 6-5 exhibited a promising inhibitory activity against SARS-CoV Mpro with IC50 of 4.471 μM and a low cellular cytotoxicity against mammalian 293 T cells. Based on the results of molecular modeling, HOMO-1 was considered to be a factor that affects AHAS inhibition and a possible binding mode of 6-5 with SARS-CoV Mpro was predicted. This is the first time that PTB derivatives have been studied as biological agents other than herbicides. The present research hence has suggested that more attentions should be paid to compounds belonging to this family to develop novel agrochemicals or medicines.
Keywords: Acetohydroxyacid synthase; Biological activity; Crystal structure; Pyrithiobac derivative; SARS-CoV M(pro).
Copyright © 2019 Elsevier Masson SAS. All rights reserved.
Figures



Similar articles
-
Chemical synthesis, in vitro acetohydroxyacid synthase (AHAS) inhibition, herbicidal activity, and computational studies of isatin derivatives.J Agric Food Chem. 2011 Sep 28;59(18):9892-900. doi: 10.1021/jf2021607. Epub 2011 Aug 25. J Agric Food Chem. 2011. PMID: 21838297
-
Design, synthesis and herbicidal activity study of aryl 2,6-disubstituted sulfonylureas as potent acetohydroxyacid synthase inhibitors.Bioorg Med Chem Lett. 2017 Aug 1;27(15):3365-3369. doi: 10.1016/j.bmcl.2017.06.007. Epub 2017 Jun 3. Bioorg Med Chem Lett. 2017. PMID: 28610985
-
Comprehensive understanding of acetohydroxyacid synthase inhibition by different herbicide families.Proc Natl Acad Sci U S A. 2017 Feb 14;114(7):E1091-E1100. doi: 10.1073/pnas.1616142114. Epub 2017 Jan 30. Proc Natl Acad Sci U S A. 2017. PMID: 28137884 Free PMC article.
-
Resistance to AHAS inhibitor herbicides: current understanding.Pest Manag Sci. 2014 Sep;70(9):1340-50. doi: 10.1002/ps.3710. Epub 2014 Jan 20. Pest Manag Sci. 2014. PMID: 24338926 Review.
-
Bacterial acetohydroxyacid synthase and its inhibitors--a summary of their structure, biological activity and current status.FEBS J. 2012 Mar;279(6):946-63. doi: 10.1111/j.1742-4658.2012.08505.x. Epub 2012 Feb 27. FEBS J. 2012. PMID: 22284339 Review.
Cited by
-
Potential of coronavirus 3C-like protease inhibitors for the development of new anti-SARS-CoV-2 drugs: Insights from structures of protease and inhibitors.Int J Antimicrob Agents. 2020 Aug;56(2):106055. doi: 10.1016/j.ijantimicag.2020.106055. Epub 2020 Jun 11. Int J Antimicrob Agents. 2020. PMID: 32534187 Free PMC article.
-
ReI Tricarbonyl Complexes as Coordinate Covalent Inhibitors for the SARS-CoV-2 Main Cysteine Protease.Angew Chem Int Ed Engl. 2021 May 3;60(19):10716-10723. doi: 10.1002/anie.202016768. Epub 2021 Mar 26. Angew Chem Int Ed Engl. 2021. PMID: 33606889 Free PMC article.
-
Therapeutic approaches against coronaviruses acute respiratory syndrome.Pharmacol Res Perspect. 2021 Feb;9(1):e00691. doi: 10.1002/prp2.691. Pharmacol Res Perspect. 2021. PMID: 33378565 Free PMC article. Review.
-
Discovery and structural optimization of 3-O-β-chacotriosyl oleanane-type triterpenoids as potent entry inhibitors of SARS-CoV-2 virus infections.Eur J Med Chem. 2021 Apr 5;215:113242. doi: 10.1016/j.ejmech.2021.113242. Epub 2021 Feb 8. Eur J Med Chem. 2021. PMID: 33588180 Free PMC article.
-
Human coronaviruses and therapeutic drug discovery.Infect Dis Poverty. 2021 Mar 16;10(1):28. doi: 10.1186/s40249-021-00812-9. Infect Dis Poverty. 2021. PMID: 33726861 Free PMC article. Review.
References
-
- Devendar P., Yang G.F. Sulfur-containing agrochemicals. Top. Curr. Chem. (Cham). 2017;375:82. - PubMed
-
- Brady J.F., Li D.C., Ishizaki H., Yang C.S. Effect of diallyl sulfide on rat liver microsomal nitrosamine metabolism and other monooxygenase activities. Cancer Res. 1988;48:5937–5940. - PubMed
-
- Song X., Li P., Li M., Yang A., Yu L., Luo L., Hu D., Song B. Synthesis and investigation of the antibacterial activity and action mechanism of 1,3,4-oxadiazole thioether derivatives. Pestic. Biochem. Physiol. 2018;147:11–19. - PubMed
-
- Huang W., Chen Q., Yang W.C., Yang G.F. Efficient synthesis and antiproliferative activity of novel thioether-substituted flavonoids. Eur. J. Med. Chem. 2013;66:161–170. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

