Immune thrombocytopenia in alemtuzumab-treated MS patients: Incidence, detection, and management
- PMID: 30785358
- PMCID: PMC6950888
- DOI: 10.1177/1352458518816612
Immune thrombocytopenia in alemtuzumab-treated MS patients: Incidence, detection, and management
Abstract
Background: Alemtuzumab is a highly effective therapy for relapsing-remitting multiple sclerosis (RRMS), and immune thrombocytopenia (ITP) has been identified as a risk.
Objective: To examine ITP incidence, treatment, and outcomes during the clinical development of alemtuzumab for RRMS and discuss postmarketing experience outside clinical trials.
Methods: CAMMS223 and Comparison of Alemtuzumab and Rebif® Efficacy in Multiple Sclerosis (CARE-MS) I and II investigated two annual courses of alemtuzumab 12 mg (or 24 mg in CAMMS223/CARE-MS II) versus subcutaneous interferon beta-1a three times per week. Patients completing core studies could enroll in an extension. Monthly monitoring for ITP continued until 48 months after the last alemtuzumab infusion.
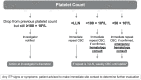
Results: Of 1485 alemtuzumab-treated MS patients in the clinical development program, 33 (2.2%) developed ITP (alemtuzumab 12 mg, 24 [2.0%]; alemtuzumab 24 mg, 9 [3.3%]) over median 6.1 years of follow-up after the first infusion; most had a sustained response to first-line ITP therapy with corticosteroids, platelets, and/or intravenous immunoglobulin. All cases occurred within 48 months of the last alemtuzumab infusion. Postmarketing surveillance data suggest that the ITP incidence is not higher in clinical practice than in clinical trials.
Conclusion: Alemtuzumab-associated ITP occurs in approximately 2% of patients and is responsive to therapy. Careful monitoring is key for detection and favorable outcomes.
Keywords: Alemtuzumab; disease-modifying therapy; immune thrombocytopenia; relapsing-remitting multiple sclerosis; safety.
Conflict of interest statement
Figures

References
-
- Brinar V, Giovannoni G, Havrdova E, et al. A phase 3b/4 long-term study of alemtuzumab in patients with relapsing-remitting multiple sclerosis: TOPAZ study design. Neurology 2015; 84: P7.219.
-
- Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: A randomised controlled phase 3 trial. Lancet 2012; 380: 1819–1828. - PubMed
-
- Coles AJ, Compston DA, Selmaj KW, et al. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med 2008; 359: 1786–1801. - PubMed
-
- Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: A randomised controlled phase 3 trial. Lancet 2012; 380: 1829–1839. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

