Improving Provider Adoption With Adaptive Clinical Decision Support Surveillance: An Observational Study
- PMID: 30785410
- PMCID: PMC6401673
- DOI: 10.2196/10245
Improving Provider Adoption With Adaptive Clinical Decision Support Surveillance: An Observational Study
Abstract
Background: Successful clinical decision support (CDS) tools can help use evidence-based medicine to effectively improve patient outcomes. However, the impact of these tools has been limited by low provider adoption due to overtriggering, leading to alert fatigue. We developed a tracking mechanism for monitoring trigger (percent of total visits for which the tool triggers) and adoption (percent of completed tools) rates of a complex CDS tool based on the Wells criteria for pulmonary embolism (PE).
Objective: We aimed to monitor and evaluate the adoption and trigger rates of the tool and assess whether ongoing tool modifications would improve adoption rates.
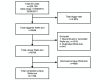
Methods: As part of a larger clinical trial, a CDS tool was developed using the Wells criteria to calculate pretest probability for PE at 2 tertiary centers' emergency departments (EDs). The tool had multiple triggers: any order for D-dimer, computed tomography (CT) of the chest with intravenous contrast, CT pulmonary angiography (CTPA), ventilation-perfusion scan, or lower extremity Doppler ultrasound. A tracking dashboard was developed using Tableau to monitor real-time trigger and adoption rates. Based on initial low provider adoption rates of the tool, we conducted small focus groups with key ED providers to elicit barriers to tool use. We identified overtriggering of the tool for non-PE-related evaluations and inability to order CT testing for intermediate-risk patients. Thus, the tool was modified to allow CT testing for the intermediate-risk group and not to trigger for CT chest with intravenous contrast orders. A dialogue box, "Are you considering PE for this patient?" was added before the tool triggered to account for CTPAs ordered for aortic dissection evaluation.
Results: In the ED of tertiary center 1, 95,295 patients visited during the academic year. The tool triggered for an average of 509 patients per month (average trigger rate 2036/30,234, 6.73%) before the modifications, reducing to 423 patients per month (average trigger rate 1629/31,361, 5.22%). In the ED of tertiary center 2, 88,956 patients visited during the academic year, with the tool triggering for about 473 patients per month (average trigger rate 1892/29,706, 6.37%) before the modifications and for about 400 per month (average trigger rate 1534/30,006, 5.12%) afterward. The modifications resulted in a significant 4.5- and 3-fold increase in provider adoption rates in tertiary centers 1 and 2, respectively. The modifications increased the average monthly adoption rate from 23.20/360 (6.5%) tools to 81.60/280.20 (29.3%) tools and 46.60/318.80 (14.7%) tools to 111.20/263.40 (42.6%) tools in centers 1 and 2, respectively.
Conclusions: Close postimplementation monitoring of CDS tools may help improve provider adoption. Adaptive modifications based on user feedback may increase targeted CDS with lower trigger rates, reducing alert fatigue and increasing provider adoption. Iterative improvements and a postimplementation monitoring dashboard can significantly improve adoption rates.
Keywords: clinical decision support; evidence-based medicine; pulmonary embolism.
©Sundas Khan, Safiya Richardson, Andrew Liu, Vinodh Mechery, Lauren McCullagh, Andy Schachter, Salvatore Pardo, Thomas McGinn. Originally published in JMIR Human Factors (http://humanfactors.jmir.org), 20.02.2019.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures





Similar articles
-
Yield of CT Pulmonary Angiography in the Emergency Department When Providers Override Evidence-based Clinical Decision Support.Radiology. 2017 Mar;282(3):717-725. doi: 10.1148/radiol.2016151985. Epub 2016 Sep 30. Radiology. 2017. PMID: 27689922 Free PMC article.
-
Usability Testing of a Complex Clinical Decision Support Tool in the Emergency Department: Lessons Learned.JMIR Hum Factors. 2015 Sep 10;2(2):e14. doi: 10.2196/humanfactors.4537. JMIR Hum Factors. 2015. PMID: 27025540 Free PMC article.
-
Higher Imaging Yield When Clinical Decision Support Is Used.J Am Coll Radiol. 2020 Apr;17(4):496-503. doi: 10.1016/j.jacr.2019.11.021. Epub 2019 Dec 30. J Am Coll Radiol. 2020. PMID: 31899178 Free PMC article.
-
Use of Computed Tomography Pulmonary Angiography in Emergency Departments: A Literature Review.Healthcare (Basel). 2022 Apr 19;10(5):753. doi: 10.3390/healthcare10050753. Healthcare (Basel). 2022. PMID: 35627890 Free PMC article. Review.
-
A critical appraisal of non-invasive diagnosis and exclusion of deep vein thrombosis and pulmonary embolism in outpatients with suspected deep vein thrombosis or pulmonary embolism: how many tests do we need?Int Angiol. 2005 Mar;24(1):27-39. Int Angiol. 2005. PMID: 15876996 Review.
Cited by
-
Clinical Decision Support and Implications for the Clinician Burnout Crisis.Yearb Med Inform. 2020 Aug;29(1):145-154. doi: 10.1055/s-0040-1701986. Epub 2020 Aug 21. Yearb Med Inform. 2020. PMID: 32823308 Free PMC article. Review.
-
A Sociotechnical Systems Framework for the Application of Artificial Intelligence in Health Care Delivery.J Cogn Eng Decis Mak. 2022 Dec;16(4):194-206. doi: 10.1177/15553434221097357. Epub 2022 May 11. J Cogn Eng Decis Mak. 2022. PMID: 36704421 Free PMC article.
-
External Validation and Refinement of Emergency Heart Failure Mortality Risk Grade Risk Model in Patients With Heart Failure in the Emergency Department.CJC Open. 2019 Apr 12;1(3):123-130. doi: 10.1016/j.cjco.2019.03.003. eCollection 2019 May. CJC Open. 2019. PMID: 32159095 Free PMC article.
-
Use, Perceived Usability, and Barriers to Implementation of a Patient Safety Dashboard Integrated within a Vendor EHR.Appl Clin Inform. 2020 Jan;11(1):34-45. doi: 10.1055/s-0039-3402756. Epub 2020 Jan 15. Appl Clin Inform. 2020. PMID: 31940670 Free PMC article.
-
Usability of a Human Factors-based Clinical Decision Support in the Emergency Department: Lessons Learned for Design and Implementation.Hum Factors. 2024 Mar;66(3):647-657. doi: 10.1177/00187208221078625. Epub 2022 Apr 14. Hum Factors. 2024. PMID: 35420923 Free PMC article.
References
-
- Randolph AG, Haynes RB, Wyatt JC, Cook DJ, Guyatt GH. Users' Guides to the Medical Literature: XVIII. How to use an article evaluating the clinical impact of a computer-based clinical decision support system. JAMA. 1999 Jul 07;282(1):67–74. - PubMed
-
- Bernstein SL, Whitaker D, Winograd J, Brennan JA. An electronic chart prompt to decrease proprietary antibiotic prescription to self-pay patients. Acad Emerg Med. 2005 Mar;12(3):225–31. doi: 10.1197/j.aem.2004.09.021. https://onlinelibrary.wiley.com/resolve/openurl?genre=article&sid=nlm:pu... - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

