Efficacy and Posttreatment Effects of Therapist-Delivered Cognitive Behavioral Therapy vs Supportive Psychotherapy for Adults With Body Dysmorphic Disorder: A Randomized Clinical Trial
- PMID: 30785624
- PMCID: PMC6450292
- DOI: 10.1001/jamapsychiatry.2018.4156
Efficacy and Posttreatment Effects of Therapist-Delivered Cognitive Behavioral Therapy vs Supportive Psychotherapy for Adults With Body Dysmorphic Disorder: A Randomized Clinical Trial
Erratum in
-
Error in Affiliation.JAMA Psychiatry. 2019 Apr 1;76(4):447. doi: 10.1001/jamapsychiatry.2019.0606. JAMA Psychiatry. 2019. PMID: 30942849 Free PMC article. No abstract available.
Abstract
Importance: Cognitive behavioral therapy (CBT), the best-studied treatment for body dysmorphic disorder (BDD), has to date not been compared with therapist-delivered supportive psychotherapy, the most commonly received psychosocial treatment for BDD.
Objective: To determine whether CBT for BDD (CBT-BDD) is superior to supportive psychotherapy in reducing BDD symptom severity and associated BDD-related insight, depressive symptoms, functional impairment, and quality of life, and whether these effects are durable.
Design, setting, and participants: This randomized clinical trial conducted at Massachusetts General Hospital and Rhode Island Hospital recruited adults with BDD between October 24, 2011, and July 7, 2016. Participants (n = 120) were randomized to the CBT-BDD arm (n = 61) or the supportive psychotherapy arm (n = 59). Weekly treatments were administered at either hospital for 24 weeks, followed by 3- and 6-month follow-up assessments. Measures were administered by blinded independent raters. Intention-to-treat statistical analyses were performed from February 9, 2017, to September 22, 2018.
Interventions: Cognitive behavioral therapy for BDD, a modular skills-based treatment, addresses the unique symptoms of the disorder. Supportive psychotherapy is a nondirective therapy that emphasizes the therapeutic relationship and self-esteem; supportive psychotherapy was enhanced with BDD-specific psychoeducation and treatment rationale.
Main outcomes and measures: The primary outcome was BDD symptom severity measured by the change in score on the Yale-Brown Obsessive-Compulsive Scale Modified for BDD from baseline to end of treatment. Secondary outcomes were the associated symptoms and these were assessed using the Brown Assessment of Beliefs Scale, Beck Depression Inventory-Second Edition, Sheehan Disability Scale, and Quality of Life Enjoyment and Satisfaction Questionnaire-Short Form.
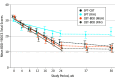
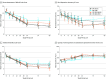
Results: Of the 120 participants, 92 (76.7%) were women, with a mean (SD) age of 34.0 (13.1) years. The difference in effectiveness between CBT-BDD and supportive psychotherapy was site specific: at 1 site, no difference was detected (estimated mean [SE] slopes, -18.6 [1.9] vs -16.7 [1.9]; P = .48; d growth-modeling analysis change, -0.25), whereas at the other site, CBT-BDD led to greater reductions in BDD symptom severity, compared with supportive psychotherapy (estimated mean [SE] slopes, -18.6 [2.2] vs -7.6 [2.0]; P < .001; d growth-modeling analysis change, -1.36). No posttreatment symptom changes were observed throughout the 6 -months of follow-up (all slope P ≥ .10).
Conclusions and relevance: Body dysmorphic disorder severity and associated symptoms appeared to improve with both CBT-BDD and supportive psychotherapy, although CBT-BDD was associated with more consistent improvement in symptom severity and quality of life.
Trial registration: ClinicalTrials.gov identifier: NCT01453439.
Conflict of interest statement
Figures

Comment in
-
Identifying Efficacious Treatment Elements for Refractory Conditions, Such as Body Dysmorphic Disorder.JAMA Psychiatry. 2019 Apr 1;76(4):357-358. doi: 10.1001/jamapsychiatry.2018.4084. JAMA Psychiatry. 2019. PMID: 30785632 No abstract available.
Similar articles
-
Modular cognitive-behavioral therapy for body dysmorphic disorder: a randomized controlled trial.Behav Ther. 2014 May;45(3):314-27. doi: 10.1016/j.beth.2013.12.007. Epub 2013 Dec 29. Behav Ther. 2014. PMID: 24680228 Free PMC article. Clinical Trial.
-
Therapist guided internet based cognitive behavioural therapy for body dysmorphic disorder: single blind randomised controlled trial.BMJ. 2016 Feb 2;352:i241. doi: 10.1136/bmj.i241. BMJ. 2016. PMID: 26837684 Free PMC article. Clinical Trial.
-
Efficacy of an internet-based, therapist-guided cognitive behavioral therapy intervention for adolescents and young adults with body dysmorphic disorder: a randomized controlled trial.BMC Psychiatry. 2025 Apr 14;25(1):374. doi: 10.1186/s12888-025-06797-1. BMC Psychiatry. 2025. PMID: 40229798 Free PMC article. Clinical Trial.
-
Is cognitive behavioral therapy an efficacious treatment for psychological interventions in body dysmorphic disorders? A meta-analysis based on current evidence from randomized controlled trials.J Affect Disord. 2024 May 1;352:237-249. doi: 10.1016/j.jad.2024.02.004. Epub 2024 Feb 16. J Affect Disord. 2024. PMID: 38369262 Review.
-
Cognitive-behavioral therapy for youth with body dysmorphic disorder: current status and future directions.Child Adolesc Psychiatr Clin N Am. 2011 Apr;20(2):287-304. doi: 10.1016/j.chc.2011.01.004. Child Adolesc Psychiatr Clin N Am. 2011. PMID: 21440856 Free PMC article. Review.
Cited by
-
An empirically derived recommendation for the classification of body dysmorphic disorder: Findings from structural equation modeling.PLoS One. 2020 Jun 3;15(6):e0233153. doi: 10.1371/journal.pone.0233153. eCollection 2020. PLoS One. 2020. PMID: 32492037 Free PMC article.
-
Predictors of pharmacotherapy outcomes for body dysmorphic disorder: a machine learning approach.Psychol Med. 2023 Jun;53(8):3366-3376. doi: 10.1017/S0033291721005390. Epub 2022 Jan 10. Psychol Med. 2023. PMID: 35000652 Free PMC article. Clinical Trial.
-
Neural and behavioral effects of modification of visual attention in body dysmorphic disorder.Transl Psychiatry. 2022 Aug 10;12(1):325. doi: 10.1038/s41398-022-02099-2. Transl Psychiatry. 2022. PMID: 35948537 Free PMC article.
-
Time to Response in Therapy for Body Dysmorphic Disorder: A Comparison of Cognitive Behavioral Therapy and Supportive Psychotherapy.Behav Ther. 2024 Jan;55(1):68-79. doi: 10.1016/j.beth.2023.05.006. Epub 2023 May 24. Behav Ther. 2024. PMID: 38216238 Free PMC article.
-
Optimizing Smartphone-Delivered Cognitive Behavioral Therapy for Body Dysmorphic Disorder Using Passive Smartphone Data: Initial Insights From an Open Pilot Trial.JMIR Mhealth Uhealth. 2020 Jun 18;8(6):e16350. doi: 10.2196/16350. JMIR Mhealth Uhealth. 2020. PMID: 32554382 Free PMC article. Clinical Trial.
References
-
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Sisorders. 5th ed. Washington, DC: American Psychiatric Pub; 2013.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

