The relationship between metastatic potential and in vitro mechanical properties of osteosarcoma cells
- PMID: 30785850
- PMCID: PMC6589788
- DOI: 10.1091/mbc.E18-08-0545
The relationship between metastatic potential and in vitro mechanical properties of osteosarcoma cells
Abstract
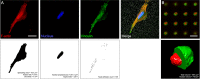
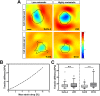
Osteosarcoma is the most frequent primary tumor of bone and is characterized by its high tendency to metastasize in lungs. Although treatment in cases of early diagnosis results in a 5-yr survival rate of nearly 60%, the prognosis for patients with secondary lesions at diagnosis is poor, and their 5-yr survival rate remains below 30%. In the present work, we have used a number of analytical methods to investigate the impact of increased metastatic potential on the biophysical properties and force generation of osteosarcoma cells. With that aim, we used two paired osteosarcoma cell lines, with each one comprising a parental line with low metastatic potential and its experimentally selected, highly metastatic form. Mechanical characterization was performed by means of atomic force microscopy, tensile biaxial deformation, and real-time deformability, and cell traction was measured using two-dimensional and micropost-based traction force microscopy. Our results reveal that the low metastatic osteosarcoma cells display larger spreading sizes and generate higher forces than the experimentally selected, highly malignant variants. In turn, the outcome of cell stiffness measurements strongly depends on the method used and the state of the probed cell, indicating that only a set of phenotyping methods provides the full picture of cell mechanics.
Figures






References
-
- Arganda-Carreras I, Sorzano COS, Marabini R, Carazo JM, Ortiz-de-Solorzano C, Kybic J. (2006). Consistent and elastic registration of histological sections using vector-spline regularization. In: Computer Vision Approaches to Medical Image Analysis, Heidelberg, Germany: Springer, 85–95.
-
- Bartalena G, Grieder R, Sharma RI, Zambelli T, Muff R, Snedeker JG. (2011). A novel method for assessing adherent single-cell stiffness in tension: design and testing of a substrate-based live cell functional imaging device. Biomed Microdevices , 291–301. - PubMed
-
- Bartalena G, Loosli Y, Zambelli T, Snedeker JG. (2012). Biomaterial surface modifications can dominate cell–substrate mechanics: the impact of PDMS plasma treatment on a quantitative assay of cell stiffness. Soft Matter , 673.
-
- Boyd NF, Rommens JM, Vogt K, Lee V, Hopper JL, Yaffe MJ, Paterson AD. (2005). Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol , 798–808. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

