A Phase 3, Randomized, Placebo-Controlled Evaluation of the Safety of Intravenous Meloxicam Following Major Surgery
- PMID: 30786162
- PMCID: PMC6899482
- DOI: 10.1002/cpdd.666
A Phase 3, Randomized, Placebo-Controlled Evaluation of the Safety of Intravenous Meloxicam Following Major Surgery
Erratum in
-
Correction.Clin Pharmacol Drug Dev. 2020 Aug;9(6):774. doi: 10.1002/cpdd.854. Epub 2020 Jul 21. Clin Pharmacol Drug Dev. 2020. PMID: 32757446 Free PMC article. No abstract available.
Abstract
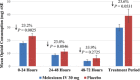
An intravenous (IV) formulation of meloxicam is being studied for moderate to severe pain management. This phase 3, randomized, multicenter, double-blind, placebo-controlled trial evaluated the safety of once-daily meloxicam IV 30 mg in subjects following major elective surgery. Eligible subjects were randomized (3:1) to receive meloxicam IV 30 mg or placebo administered once daily. Safety was evaluated via adverse events, clinical laboratory tests, vital signs, wound healing, and opioid consumption. The incidence of adverse events was similar between meloxicam IV- and placebo-treated subjects (63.0% versus 65.0%). Investigators assessed most adverse events as mild or moderate in intensity and unrelated to treatment. Adverse events of interest (injection-site reactions, bleeding, cardiovascular, hepatic, renal, thrombotic, and wound-healing events) were similar between groups. Over the treatment period, meloxicam IV was associated with a 23.6% (P = .0531) reduction in total opioid use (9.2 mg morphine equivalent) compared to placebo-treated subjects. The results suggest that meloxicam IV had a safety profile similar to that of placebo with respect to numbers and frequencies of adverse events and reduced opioid consumption in subjects with moderate to severe postoperative pain following major elective surgery.
Trial registration: ClinicalTrials.gov NCT02720692.
Keywords: meloxicam; nonsteroidal anti-inflammatory drug; postoperative pain; safety; surgery.
© 2019 The Authors. Clinical Pharmacology in Drug Development Published by Wiley Periodicals, Inc. on behalf of American College of Clinical Pharmacology.
Conflict of interest statement
Sergio D. Bergese has received grants from Recro Pharma, Inc. Keith A. Candiotti has received consultancies and grants from Recro Pharma, Inc. Randall J. Mack and Stewart W. McCallum have stock ownership and options and are employees of Recro Pharma, Inc. Wei Du receives consultancies from Recro Pharma, Inc. Alexis Gomez has stock ownership and options and was an employee of Recro Pharma, Inc at the time of this study. Timothy I. Melson, Sabry S. Ayad, and Jorge E. Marcet do not have any conflicts of interest to disclose.
Figures

References
-
- Joshi GP, Ogunnaike BO. Consequences of inadequate postoperative pain relief and chronic persistent postoperative pain. Anesthesiol Clin North Am. 2005;23(1):21‐36. - PubMed
-
- Prabhakar A, Mancuso KF, Owen CP, et al. Perioperative analgesia outcomes and strategies. Best Pract Res Clin Anaesthesiol. 2014;28(2):105‐115. - PubMed
-
- Gan TJ, Habib AS, Miller TE, White W, Apfelbaum JL. Incidence, patient satisfaction, and perceptions of post‐surgical pain: results from a US national survey. Curr Med Res Opin. 2014;30(1):149‐160. - PubMed
-
- Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97(2):534‐540. - PubMed
-
- Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician. 2008;11(2 suppl):S105‐S120. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

