Abnormal Sleep Spindles, Memory Consolidation, and Schizophrenia
- PMID: 30786245
- PMCID: PMC7307009
- DOI: 10.1146/annurev-clinpsy-050718-095754
Abnormal Sleep Spindles, Memory Consolidation, and Schizophrenia
Abstract
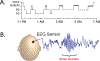
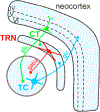
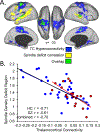
There is overwhelming evidence that sleep is crucial for memory consolidation. Patients with schizophrenia and their unaffected relatives have a specific deficit in sleep spindles, a defining oscillation of non-rapid eye movement (NREM) Stage 2 sleep that, in coordination with other NREM oscillations, mediate memory consolidation. In schizophrenia, the spindle deficit correlates with impaired sleep-dependent memory consolidation, positive symptoms, and abnormal thalamocortical connectivity. These relations point to dysfunction of the thalamic reticular nucleus (TRN), which generates spindles, gates the relay of sensory information to the cortex, and modulates thalamocortical communication. Genetic studies are beginning to provide clues to possible neurodevelopmental origins of TRN-mediated thalamocortical circuit dysfunction and to identify novel targets for treating the related memory deficits and symptoms. By forging empirical links in causal chains from risk genes to thalamocortical circuit dysfunction, spindle deficits, memory impairment, symptoms, and diagnosis, future research can advance our mechanistic understanding, treatment, and prevention of schizophrenia.
Keywords: cognition; endophenotype; genetics; memory; schizophrenia; sleep; spindles.
Figures



References
-
- Ambrosius U, Lietzenmaier S, Wehrle R, Wichniak A, Kalus S, et al. 2008. Heritability of sleep electroencephalogram. Biol Psychiatry 64: 344–8 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

