Diabetes Alters pH Control in Rat Retina
- PMID: 30786276
- PMCID: PMC6383832
- DOI: 10.1167/iovs.18-26073
Diabetes Alters pH Control in Rat Retina
Abstract
Purpose: The purpose of this study was to determine whether the ability of the rat retina to control its pH is affected by diabetes.
Methods: Double-barreled H+-selective microelectrodes were used to measure extracellular [H+] in the dark-adapted retina of intact control and diabetic Long-Evans rats 1 to 6 months after intraperitoneal injection of vehicle or streptozotocin, respectively. Two manipulations-increasing of blood glucose and intravenous injection of the carbonic anhydrase blocker dorzolamide (DZM)-were used to examine their effects on retinal pH regulation.
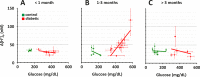
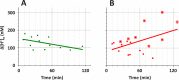
Results: An increase of retinal acidity was correlated with the diabetes-related increase in blood glucose, but only between 1 and 3 months of diabetes, not earlier or later. Adding intravenous glucose had no noticeable effect on the retinal acidity of control animals. In contrast, similar injections of glucose in diabetic rats significantly increased the acidity of the retina. Again, the largest increase of retinal acidity due to artificially elevated blood glucose was observed at 1 to 3 months of diabetes. Suppression of carbonic anhydrase by DZM dramatically increased the retinal acidity in both control and diabetic retinas to a similar degree. However, in controls, the strongest effect of DZM was recorded within 10 minutes after the injection, but in diabetics, the effect tended to increase with time and after 2 hours could be two to three times larger than at the beginning.
Conclusions: During development of diabetes in rats, the control over retinal pH is partly compromised so that conditions that perturb retinal pH lead to larger and/or more sustained changes than in control animals.
Figures





References
-
- Wang L, Tornquist P, Bill A. Glucose metabolism in pig outer retina in light and darkness. Acta Physiol Scand. 1997;160:75–81. - PubMed
-
- Winkler BS. A quantitative assessment of glucose metabolism in the isolated rat retina. In: Christen Y, Doly M, Droy-Lefaix M, editors. Les Seminaires Ophthalmologiques d'IPSEN, Vision et Adaptation. Paris: Elsevier;; 1995. pp. 78–96.
-
- Padnick-Silver L, Linsenmeier RA. Effect of hypoxemia and hyperglycemia on pH in the intact cat retina. Arch Ophthalmol. 2005;123:1684–1690. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

