Varicella Zoster Virus Induces Differential Cell-Type Specific Responses in Human Corneal Epithelial Cells and Keratocytes
- PMID: 30786281
- PMCID: PMC6383726
- DOI: 10.1167/iovs.18-25801
Varicella Zoster Virus Induces Differential Cell-Type Specific Responses in Human Corneal Epithelial Cells and Keratocytes
Abstract
Purpose: While VZV DNA and antigen have been detected in acute and chronic VZV keratitis, it is unclear whether productive infection of corneal cells is ongoing or whether residual, noninfectious VZV antigens elicit inflammation. Herein, we examined VZV-infected primary human corneal epithelial cells (HCECs) and keratocytes (HKs) to elucidate the pathogenesis of VZV keratitis.
Methods: HCECs and HKs were mock- or VZV infected. Seven days later, cells were examined for morphology, proinflammatory cytokine and matrix metalloproteinase (MMP) release, ability to recruit peripheral blood mononuclear cells (PBMCs) and neutrophils, and MMP substrate cleavage.
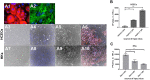
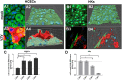
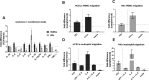
Results: Both cell types synthesized infectious virus. VZV-infected HCECs proliferated, whereas VZV-infected HKs died. Compared to mock-infected cells, VZV-infected HCECs secreted significantly more IL-6, IL-8, IL-10, and IL-12p70 that were confirmed at the transcript level, and MMP-1 and MMP-9; conditioned supernatant attracted PBMCs and neutrophils and cleaved MMP substrates. In contrast, VZV-infected HKs suppressed cytokine secretion except for IL-8, which attracted neutrophils, and suppressed MMP release and substrate cleavage.
Conclusions: Overall, VZV-infected HCECs recapitulate findings of VZV keratitis with respect to epithelial cell proliferation, pseudodendrite formation and creation of a proinflammatory environment, providing an in vitro model for VZV infection of corneal epithelial cells. Furthermore, the proliferation and persistence of VZV-infected HCECs suggest that these cells may serve as viral reservoirs if immune clearance is incomplete. Finally, the finding that VZV-infected HKs die and suppress most proinflammatory cytokines and MMPs may explain the widespread death of these cells with unchecked viral spread due to ineffective recruitment of PBMCs.
Figures
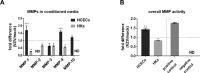
References
-
- Ragozzino MW, Melton LJ, III, Kurland LT, Chu CP, Perry HO. Population-based study of herpes zoster and its sequelae. Medicine (Baltimore) 1982;61:310–316. - PubMed
-
- Liesegang TJ. Herpes zoster ophthalmicus natural history, risk factors, clinical presentation, and morbidity. Ophthalmology. 2008;115:S3–S12. - PubMed
-
- Szeto SK, Chan TC, Wong RL, Ng AL, Li EY, Jhanji V. Prevalence of ocular manifestations and visual outcomes in patients with herpes zoster ophthalmicus. Cornea. 2017;36:338–342. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

