Effectiveness of Insulin Analogs Compared with Human Insulins in Pregnant Women with Diabetes Mellitus: Systematic Review and Meta-analysis
- PMID: 30786308
- PMCID: PMC10418821
- DOI: 10.1055/s-0038-1676510
Effectiveness of Insulin Analogs Compared with Human Insulins in Pregnant Women with Diabetes Mellitus: Systematic Review and Meta-analysis
Abstract
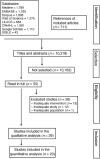
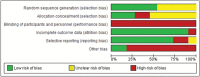
Diabetes during pregnancy has been linked to unfavorable maternal-fetal outcomes. Human insulins are the first drug of choice because of the proven safety in their use. However, there are still questions about the use of insulin analogs during pregnancy. The objective of the present study was to determine the effectiveness of insulin analogs compared with human insulin in the treatment of pregnant women with diabetes through a systematic review with meta-analysis. The search comprised the period since the inception of each database until July 2017, and the following databases were used: MEDLINE, CINAHL, EMBASE, ISI Web of Science, LILACS, Scopus, SIGLE and Google Scholar. We have selected 29 original articles: 11 were randomized clinical trials and 18 were observational studies. We have explored data from 6,382 participants. All of the articles were classified as having an intermediate to high risk of bias. The variable that showed favorable results for the use of insulin analogs was gestational age, with a mean difference of - 0.26 (95 % confidence interval [CI]: 0.03-0.49; p = 0.02), but with significant heterogeneity (Higgins test [I2] = 38%; chi-squared test [χ2] = 16.24; degree of freedom [DF] = 10; p = 0.09). This result, in the clinical practice, does not compromise the fetal well-being, since all babies were born at term. There was publication bias in the gestational age and neonatal weight variables. To date, the evidence analyzed has a moderate-to-high risk of bias and does not allow the conclusion that insulin analogs are more effective when compared with human insulin to treat diabetic pregnant women.
Diabetes durante a gestação tem sido relacionado a desfechos materno-fetais desfavoráveis. As insulinas humanas são a primeira escolha medicamentosa, devido à comprovada segurança no seu uso. Entretanto, ainda há questionamentos sobre o uso dos análogos da insulina na gestação. O objetivo do presente estudo foi determinar a efetividade dos análogos da insulina comparados às insulinas humanas no tratamento de gestantes com diabetes por meio de uma revisão sistemática com metanálise. A busca compreendeu desde o início de cada base de dados até julho de 2017, e foi realizada nos seguintes bancos de dados: MEDLINE, CINAHL, EMBASE, ISI Web of Science, LILACS, Scopus, SIGLE e Google Scholar. Selecionamos 29 artigos originais, sendo 11 ensaios clínicos randomizados e 18 estudos observacionais. Exploramos dados de 6.382 participantes. Todos os artigos foram classificados como sendo de intermediário a alto risco de viés. A variável que demonstrou resultado favorável ao uso dos análogos da insulina foi idade gestacional, com uma diferença média de - 0.26 (95% índice de confiança [IC]: 0.03–0.49; p = 0.02), porém com heterogeneidade significativa (teste de Higgins [I2] = 38%; teste do qui quadrado [χ2] =16.24; graus de liberdade [GL] =10; p = 0.09). Esse resultado, na prática clínica, não compromete o bem-estar fetal, uma vez que todos os bebês nasceram a termo. Houve viés de publicação nas variáveis idade gestacional e peso neonatal. Até o momento, as evidências analisadas possuem um risco de viés moderado a elevado e não permitem concluir que os análogos da insulina sejam mais efetivos em comparação às insulinas humanas para tratar gestantes diabéticas.
Thieme Revinter Publicações Ltda Rio de Janeiro, Brazil.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

References
-
- International Diabetes Federation. IDF Diabetes Atlas. 8th ed Brussels: IDF; 2017. http://www.idf.org/diabetesatlas. Accessed December 1, 2017
-
- Buchanan T A, Xiang A, Kjos S L, Watanabe R.What is gestational diabetes? Diabetes Care 20073002S105–S111.. Doi: 10.2337/dc07-s201 - PubMed
-
- Ray J G, O'Brien T E, Chan W S. Preconception care and the risk of congenital anomalies in the offspring of women with diabetes mellitus: a meta-analysis. QJM. 2001;94(08):435–444. - PubMed
-
- Fetita L S, Sobngwi E, Serradas P, Calvo F, Gautier J F.Consequences of fetal exposure to maternal diabetes in offspring J Clin Endocrinol Metab 200691103718–3724.. Doi: 10.1210/jc.2006-0624 - PubMed
-
- Galerneau F, Inzucchi S E.Diabetes mellitus in pregnancy Obstet Gynecol Clin North Am 20043104907–933., xi–xii. Doi: 10.1016/j.ogc.2004.09.002 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
