Mitophagy Is Essential for Maintaining Cardiac Function During High Fat Diet-Induced Diabetic Cardiomyopathy
- PMID: 30786833
- PMCID: PMC6483841
- DOI: 10.1161/CIRCRESAHA.118.314607
Mitophagy Is Essential for Maintaining Cardiac Function During High Fat Diet-Induced Diabetic Cardiomyopathy
Abstract
Rationale: Diabetic patients develop cardiomyopathy characterized by hypertrophy, diastolic dysfunction, and intracellular lipid accumulation, termed lipotoxicity. Diabetic hearts utilize fatty acids as a major energy source, which produces high levels of oxidative stress, thereby inducing mitochondrial dysfunction.
Objective: To elucidate how mitochondrial function is regulated in diabetic cardiomyopathy.
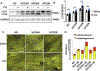
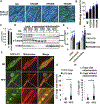
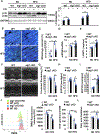
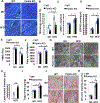
Methods and results: Mice were fed either a normal diet or high-fat diet (HFD, 60 kcal % fat). Although autophagic flux was activated by HFD consumption, peaking at 6 weeks ( P<0.05), it was attenuated thereafter. Mitophagy, evaluated with Mito-Keima, was increased after 3 weeks of HFD feeding (mitophagy area: 8.3% per cell with normal diet and 12.4% with HFD) and continued to increase even after 2 months ( P<0.05). By isolating adult cardiomyocytes from GFP-LC3 mice fed HFD, we confirmed that mitochondria were sequestrated by LC3-positive autophagosomes during mitophagy. In wild-type mice, cardiac hypertrophy, diastolic dysfunction (end diastolic pressure-volume relationship =0.051±0.009 in normal diet and 0.11±0.004 in HFD) and lipid accumulation occurred within 2 months of HFD feeding ( P<0.05). Deletion of atg7 impaired mitophagy, increased lipid accumulation, exacerbated diastolic dysfunction (end diastolic pressure-volume relationship =0.11±0.004 in wild type and 0.152±0.019 in atg7 cKO; P<0.05) and induced systolic dysfunction (end systolic pressure-volume relationship =24.86±2.46 in wild type and 15.93±1.76 in atg7 cKO; P<0.05) during HFD feeding. Deletion of Parkin partially inhibited mitophagy, increased lipid accumulation and exacerbated diastolic dysfunction (end diastolic pressure-volume relationship =0.124±0.005 in wild type and 0.176±0.018 in Parkin KO, P<0.05) in response to HFD feeding. Injection of TB1 (Tat-Beclin1) activated mitophagy, attenuated mitochondrial dysfunction, decreased lipid accumulation, and protected against cardiac diastolic dysfunction (end diastolic pressure-volume relationship =0.110±0.009 in Control peptide and 0.078±0.015 in TB1, P<0.05) during HFD feeding.
Conclusions: Mitophagy serves as an essential quality control mechanism for mitochondria in the heart during HFD consumption. Impairment of mitophagy induces mitochondrial dysfunction and lipid accumulation, thereby exacerbating diabetic cardiomyopathy. Conversely, activation of mitophagy protects against HFD-induced diabetic cardiomyopathy.
Keywords: autophagy; diabetic cardiomyopathy; fatty acids; mitochondria; oxidative stress.
Figures


Comment in
-
Activation of Mitophagy in High-Fat Diet-Induced Diabetic Cardiomyopathy.Circ Res. 2019 Apr 26;124(9):1288-1290. doi: 10.1161/CIRCRESAHA.119.314967. Circ Res. 2019. PMID: 31021731 No abstract available.
References
-
- Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, Salama JS, Vos T, Abate KH, Abbafati C, Ahmed MB, Al-Aly Z, Alkerwi A, Al-Raddadi R, Amare AT, Amberbir A, Amegah AK, Amini E, Amrock SM, Anjana RM, Arnlov J, Asayesh H, Banerjee A, Barac A, Baye E, Bennett DA, Beyene AS, Biadgilign S, Biryukov S, Bjertness E, Boneya DJ, Campos-Nonato I, Carrero JJ, Cecilio P, Cercy K, Ciobanu LG, Cornaby L, Damtew SA, Dandona L, Dandona R, Dharmaratne SD, Duncan BB, Eshrati B, Esteghamati A, Feigin VL, Fernandes JC, Furst T, Gebrehiwot TT, Gold A, Gona PN, Goto A, Habtewold TD, Hadush KT, Hafezi-Nejad N, Hay SI, Horino M, Islami F, Kamal R, Kasaeian A, Katikireddi SV, Kengne AP, Kesavachandran CN, Khader YS, Khang YH, Khubchandani J, Kim D, Kim YJ, Kinfu Y, Kosen S, Ku T, Defo BK, Kumar GA, Larson HJ, Leinsalu M, Liang X, Lim SS, Liu P, Lopez AD, Lozano R, Majeed A, Malekzadeh R, Malta DC, Mazidi M, McAlinden C, McGarvey ST, Mengistu DT, Mensah GA, Mensink GBM, Mezgebe HB, Mirrakhimov EM, Mueller UO, Noubiap JJ, Obermeyer CM, Ogbo FA, Owolabi MO, Patton GC, Pourmalek F, Qorbani M, Rafay A, Rai RK, Ranabhat CL, Reinig N, Safiri S, Salomon JA, Sanabria JR, Santos IS, Sartorius B, Sawhney M, Schmidhuber J, Schutte AE, Schmidt MI, Sepanlou SG, Shamsizadeh M, Sheikhbahaei S, Shin MJ, Shiri R, Shiue I, Roba HS, Silva DAS, Silverberg JI, Singh JA, Stranges S, Swaminathan S, Tabares-Seisdedos R, Tadese F, Tedla BA, Tegegne BS, Terkawi AS, Thakur JS, Tonelli M, Topor-Madry R, Tyrovolas S, Ukwaja KN, Uthman OA, Vaezghasemi M, Vasankari T, Vlassov VV, Vollset SE, Weiderpass E, Werdecker A, Wesana J, Westerman R, Yano Y, Yonemoto N, Yonga G, Zaidi Z, Zenebe ZM, Zipkin B and Murray CJL. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. The New England journal of medicine. 2017;377:13–27. - PMC - PubMed
-
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ and Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2013;62:e147–239. - PubMed
-
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW and Westlake C. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

